Amelioration of Clostridium difficile Infection in Mice by Dietary Supplementation With Indole-3-carbinol
- PMID: 27280500
- PMCID: PMC5743052
- DOI: 10.1097/SLA.0000000000001830
Amelioration of Clostridium difficile Infection in Mice by Dietary Supplementation With Indole-3-carbinol
Abstract
Objective: To determine the therapeutic effects of dietary supplementation on Clostridium difficile infection (CDI).
Background: With limited treatment options, the rise of C. difficile-associated disease has spurred on the search for novel therapies. Recent data define a role for the aryl hydrocarbon receptor (AHR) and diet-derived AHR ligands in mucosal immunity. We investigated the efficacy of indole-3-carbinol (I3C), a dietary supplement, and AHR precursor ligand in a murine model of CDI.
Methods: C57BL/6 (B6), AHR, and AHR mice were placed on either grain-based or semipurified diets with or without I3C before and during CDI. Mice were followed clinically for a minimum of 6 days or euthanized between days 0 and 4 of inoculation for analysis of the inflammatory response and microbiota.
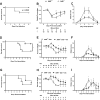
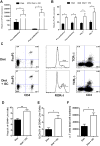
Results: B6 mice fed an AHR ligand-deficient, semipurified diet have significantly increased disease severity (P<0.001) and mortality (P < 0.001) compared with mice fed on diet containing I3C. The addition of I3C to the diet of AHR null mice had less of an impact than in AHR heterozygous littermates, although some protection was seen. Mice on semipurified I3C-diet had increased cecal Tregs, ILC3s, and γδ T cells and an increased neutrophilic response without increased inflammation or bacterial translocation compared with controls.
Conclusions: I3C is a powerful treatment to reduce impact of CDI in mice. The findings indicate I3C may be acting through both AHR-dependent and -independent mechanisms in this model. Dietary supplementation with I3C is a potential new therapy for prevention and amelioration of C. difficile disease.
Figures


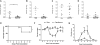
Similar articles
-
Dietary Indole-3-Carbinol Activates AhR in the Gut, Alters Th17-Microbe Interactions, and Exacerbates Insulitis in NOD Mice.Front Immunol. 2021 Jan 21;11:606441. doi: 10.3389/fimmu.2020.606441. eCollection 2020. Front Immunol. 2021. PMID: 33552063 Free PMC article.
-
Dietary AhR Ligands Regulate AhRR Expression in Intestinal Immune Cells and Intestinal Microbiota Composition.Int J Mol Sci. 2020 Apr 30;21(9):3189. doi: 10.3390/ijms21093189. Int J Mol Sci. 2020. PMID: 32366032 Free PMC article.
-
Potential Dietary and Therapeutic Strategies Involving Indole-3-Carbinole in Preclinical Models of Intestinal Inflammation.Nutrients. 2023 Nov 30;15(23):4980. doi: 10.3390/nu15234980. Nutrients. 2023. PMID: 38068838 Free PMC article.
-
Indole-3-carbinol induces tumor cell death: function follows form.J Surg Res. 2016 Jul;204(1):47-54. doi: 10.1016/j.jss.2016.04.021. Epub 2016 Apr 22. J Surg Res. 2016. PMID: 27451867 Free PMC article. Review.
-
Clostridium Difficile Infection: An Immunological Conundrum.Arch Med Res. 2018 Aug;49(6):359-364. doi: 10.1016/j.arcmed.2018.11.002. Epub 2019 Jan 4. Arch Med Res. 2018. PMID: 30617004 Review.
Cited by
-
Cancer-Associated Microbiota: From Mechanisms of Disease Causation to Microbiota-Centric Anti-Cancer Approaches.Biology (Basel). 2022 May 16;11(5):757. doi: 10.3390/biology11050757. Biology (Basel). 2022. PMID: 35625485 Free PMC article. Review.
-
Ambient urban dust particulate matter reduces pathologic T cells in the CNS and severity of EAE.Environ Res. 2019 Jan;168:178-192. doi: 10.1016/j.envres.2018.09.038. Epub 2018 Oct 1. Environ Res. 2019. PMID: 30316103 Free PMC article.
-
Dietary Indole-3-Carbinol Activates AhR in the Gut, Alters Th17-Microbe Interactions, and Exacerbates Insulitis in NOD Mice.Front Immunol. 2021 Jan 21;11:606441. doi: 10.3389/fimmu.2020.606441. eCollection 2020. Front Immunol. 2021. PMID: 33552063 Free PMC article.
-
Type 3 Innate Lymphoid Cells as Regulators of the Host-Pathogen Interaction.Front Immunol. 2021 Sep 29;12:748851. doi: 10.3389/fimmu.2021.748851. eCollection 2021. Front Immunol. 2021. PMID: 34659248 Free PMC article. Review.
-
Indoles Derived From Glucobrassicin: Cancer Chemoprevention by Indole-3-Carbinol and 3,3'-Diindolylmethane.Front Nutr. 2021 Oct 1;8:734334. doi: 10.3389/fnut.2021.734334. eCollection 2021. Front Nutr. 2021. PMID: 34660663 Free PMC article. Review.
References
-
- Surawicz CM. Clostridium difficile infection: risk factors, diagnosis and management. Curr Treat Options Gastroenterol. 2015;13(1):121–9. - PubMed
-
- Regnault H, Bourrier A, Lalande V, et al. Prevalence and risk factors of Clostridium difficile infection in patients hospitalized for flare of inflammatory bowel disease: a retrospective assessment. Dig Liver Dis. 2014;46(12):1086–92. - PubMed
-
- Biswal S. Proton pump inhibitors and risk for Clostridium difficile associated diarrhea. Biomed J. 2014;37(4):178–83. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

