A universal system to select gene-modified hepatocytes in vivo
- PMID: 27280686
- PMCID: PMC5242329
- DOI: 10.1126/scitranslmed.aad8166
A universal system to select gene-modified hepatocytes in vivo
Abstract
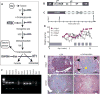
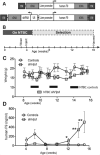
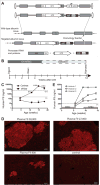
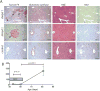
Many genetic and acquired liver disorders are amenable to gene and/or cell therapy. However, the efficiencies of cell engraftment and stable genetic modification are low and often subtherapeutic. In particular, targeted gene modifications from homologous recombination are rare events. These obstacles could be overcome if hepatocytes that have undergone genetic modification were to be selectively amplified or expanded. We describe a universally applicable system for in vivo selection and expansion of gene-modified hepatocytes in any genetic background. In this system, the therapeutic transgene is coexpressed with a short hairpin RNA (shRNA) that confers modified hepatocytes with resistance to drug-induced toxicity. An shRNA against the tyrosine catabolic enzyme 4-OH-phenylpyruvate dioxygenase protected hepatocytes from 4-[(2-carboxyethyl)-hydroxyphosphinyl]-3-oxobutyrate, a small-molecule inhibitor of fumarylacetoacetate hydrolase. To select for specific gene targeting events, the protective shRNA was embedded in a microRNA and inserted into a recombinant adeno-associated viral vector designed to integrate site-specifically into the highly active albumin locus. After selection of the gene-targeted cells, transgene expression increased 10- to 1000-fold, reaching supraphysiological levels of human factor 9 protein (50,000 ng/ml) in mice. This drug resistance system can be used to achieve therapeutically relevant transgene levels in hepatocytes in any setting.
Copyright © 2016, American Association for the Advancement of Science.
Figures


References
-
- Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341–355. - PubMed
-
- Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O'Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CY, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, Nienhuis AW, Davidoff AM. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–2365. - PMC - PubMed
-
- Pien GC, Basner-Tschakarjan E, Hui DJ, Mentlik AN, Finn JD, Hasbrouck NC, Zhou S, Murphy SL, Maus MV, Mingozzi F, Orange JS, High KA. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest. 2009;119:1688–1695. - PMC - PubMed
-
- Mingozzi F, High KA. Immune responses to AAV in clinical trials. Curr Gene Ther. 2007;7:316–324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

