Extratumoral Heme Oxygenase-1 (HO-1) Expressing Macrophages Likely Promote Primary and Metastatic Prostate Tumor Growth
- PMID: 27280718
- PMCID: PMC4900522
- DOI: 10.1371/journal.pone.0157280
Extratumoral Heme Oxygenase-1 (HO-1) Expressing Macrophages Likely Promote Primary and Metastatic Prostate Tumor Growth
Abstract
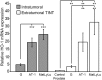
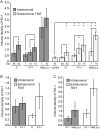
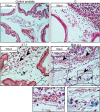
Aggressive tumors induce tumor-supporting changes in the benign parts of the prostate. One factor that has increased expression outside prostate tumors is hemoxygenase-1 (HO-1). To investigate HO-1 expression in more detail, we analyzed samples of tumor tissue and peritumoral normal prostate tissue from rats carrying cancers with different metastatic capacity, and human prostate cancer tissue samples from primary tumors and bone metastases. In rat prostate tumor samples, immunohistochemistry and quantitative RT-PCR showed that the main site of HO-1 synthesis was HO-1+ macrophages that accumulated in the tumor-bearing organ, and at the tumor-invasive front. Small metastatic tumors were considerably more effective in attracting HO-1+ macrophages than larger non-metastatic ones. In clinical samples, accumulation of HO-1+ macrophages was seen at the tumor invasive front, almost exclusively in high-grade tumors, and it correlated with the presence of bone metastases. HO-1+ macrophages, located at the tumor invasive front, were more abundant in bone metastases than in primary tumors. HO-1 expression in bone metastases was variable, and positively correlated with the expression of macrophage markers but negatively correlated with androgen receptor expression, suggesting that elevated HO-1 could be a marker for a subgroup of bone metastases. Together with another recent observation showing that selective knockout of HO-1 in macrophages reduced prostate tumor growth and metastatic capacity in animals, the results of this study suggest that extratumoral HO-1+ macrophages may have an important role in prostate cancer.
Conflict of interest statement
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

