Treatment With Human Wharton's Jelly-Derived Mesenchymal Stem Cells Attenuates Sepsis-Induced Kidney Injury, Liver Injury, and Endothelial Dysfunction
- PMID: 27280799
- PMCID: PMC4954445
- DOI: 10.5966/sctm.2015-0138
Treatment With Human Wharton's Jelly-Derived Mesenchymal Stem Cells Attenuates Sepsis-Induced Kidney Injury, Liver Injury, and Endothelial Dysfunction
Abstract
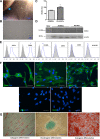
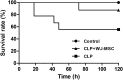
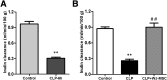
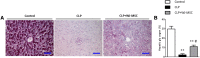
: The pathophysiology of sepsis involves complex cytokine and inflammatory mediator networks. Downregulation of endothelial nitric oxide synthase contributes to sepsis-induced endothelial dysfunction. Human Wharton's jelly-derived mesenchymal stem cells (WJ-MSCs) are known to reduce expression of proinflammatory cytokines and markers of apoptosis. We hypothesized that treatment with WJ-MSCs would protect renal, hepatic, and endothelial function in a cecal ligation and puncture (CLP) model of sepsis in rats. Rats were randomly divided into three groups: sham-operated rats; rats submitted to CLP and left untreated; and rats submitted to CLP and intraperitoneally injected, 6 hours later, with 1 × 10(6) WJ-MSCs. The glomerular filtration rate (GFR) was measured at 6 and 24 hours after CLP or sham surgery. All other studies were conducted at 24 hours after CLP or sham surgery. By 6 hours, GFR had decreased in the CLP rats. At 24 hours, Klotho renal expression significantly decreased. Treatment with WJ-MSCs improved the GFR; improved tubular function; decreased the CD68-positive cell count; decreased the fractional interstitial area; decreased expression of nuclear factor κB and of cytokines; increased expression of eNOS, vascular endothelial growth factor, and Klotho; attenuated renal apoptosis; ameliorated hepatic function; increased glycogen deposition in the liver; and improved survival. Sepsis-induced acute kidney injury is a state of Klotho deficiency, which WJ-MSCs can attenuate. Klotho protein expression was higher in WJ-MSCs than in human adipose-derived MSCs. Because WJ-MSCs preserve renal and hepatic function, they might play a protective role in sepsis.
Significance: Sepsis is the leading cause of death in intensive care units. Although many different treatments for sepsis have been tested, sepsis-related mortality rates remain high. It was hypothesized in this study that treatment with human Wharton's jelly-derived mesenchymal stem cells (WJ-MSCs) would protect renal, hepatic, and endothelial function in a model of sepsis in rats. Treatment with WJ-MSCs improved the glomerular filtration rate, improved tubular function, decreased expression of nuclear factor κB and of cytokines, increased expression of eNOS and of Klotho, attenuated renal apoptosis, and improved survival. Sepsis-induced acute kidney injury is a state of Klotho deficiency, which WJ-MSCs can attenuate.
Keywords: Apoptosis; Endothelium-derived factors; Interleukins; Kidney injury; Liver injury; Wharton’s jelly-derived mesenchymal stem cells.
©AlphaMed Press.
Figures


References
-
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. - PubMed
-
- Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med. 2007;35:2408–2416. - PubMed
-
- Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431–439. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

