Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy
- PMID: 27281029
- PMCID: PMC4900555
- DOI: 10.1371/journal.pone.0157164
Optimizing Timing of Immunotherapy Improves Control of Tumors by Hypofractionated Radiation Therapy
Abstract
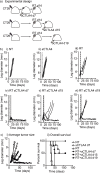
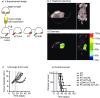
The anecdotal reports of promising results seen with immunotherapy and radiation in advanced malignancies have prompted several trials combining immunotherapy and radiation. However, the ideal timing of immunotherapy with radiation has not been clarified. Tumor bearing mice were treated with 20Gy radiation delivered only to the tumor combined with either anti-CTLA4 antibody or anti-OX40 agonist antibody. Immunotherapy was delivered at a single timepoint around radiation. Surprisingly, the optimal timing of these therapies varied. Anti-CTLA4 was most effective when given prior to radiation therapy, in part due to regulatory T cell depletion. Administration of anti-OX40 agonist antibody was optimal when delivered one day following radiation during the post-radiation window of increased antigen presentation. Combination treatment of anti-CTLA4, radiation, and anti-OX40 using the ideal timing in a transplanted spontaneous mammary tumor model demonstrated tumor cures. These data demonstrate that the combination of immunotherapy and radiation results in improved therapeutic efficacy, and that the ideal timing of administration with radiation is dependent on the mechanism of action of the immunotherapy utilized.
Conflict of interest statement
Figures


Similar articles
-
Combination OX40 agonism/CTLA-4 blockade with HER2 vaccination reverses T-cell anergy and promotes survival in tumor-bearing mice.Proc Natl Acad Sci U S A. 2016 Jan 19;113(3):E319-27. doi: 10.1073/pnas.1510518113. Epub 2016 Jan 4. Proc Natl Acad Sci U S A. 2016. PMID: 26729864 Free PMC article.
-
Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity.Cancer Immunol Res. 2014 Feb;2(2):142-53. doi: 10.1158/2326-6066.CIR-13-0031-T. Epub 2013 Nov 11. Cancer Immunol Res. 2014. PMID: 24778278 Free PMC article.
-
Administration of low-dose combination anti-CTLA4, anti-CD137, and anti-OX40 into murine tumor or proximal to the tumor draining lymph node induces systemic tumor regression.Cancer Immunol Immunother. 2018 Jan;67(1):47-60. doi: 10.1007/s00262-017-2059-y. Epub 2017 Sep 13. Cancer Immunol Immunother. 2018. PMID: 28905118 Free PMC article.
-
OX40 signaling in head and neck squamous cell carcinoma: Overcoming immunosuppression in the tumor microenvironment.Oral Oncol. 2016 Jan;52:1-10. doi: 10.1016/j.oraloncology.2015.11.009. Epub 2015 Nov 21. Oral Oncol. 2016. PMID: 26614363 Review.
-
Exploring optimal sequencing of radiation and immunotherapy combinations.Adv Radiat Oncol. 2018 Oct 23;3(4):494-505. doi: 10.1016/j.adro.2018.07.005. eCollection 2018 Oct-Dec. Adv Radiat Oncol. 2018. PMID: 30370348 Free PMC article. Review.
Cited by
-
Radiation-induced bystander and abscopal effects: important lessons from preclinical models.Br J Cancer. 2020 Aug;123(3):339-348. doi: 10.1038/s41416-020-0942-3. Epub 2020 Jun 25. Br J Cancer. 2020. PMID: 32581341 Free PMC article. Review.
-
Like a Rolling Stone: Sting-Cgas Pathway and Cell-Free DNA as Biomarkers for Combinatorial Immunotherapy.Pharmaceutics. 2020 Aug 11;12(8):758. doi: 10.3390/pharmaceutics12080758. Pharmaceutics. 2020. PMID: 32796670 Free PMC article. Review.
-
Adoptive T-Cell Therapy in Advanced Colorectal Cancer: A Systematic Review.Oncologist. 2022 Mar 11;27(3):210-219. doi: 10.1093/oncolo/oyab038. Oncologist. 2022. PMID: 35274719 Free PMC article.
-
Radiotherapy-Induced Changes in the Systemic Immune and Inflammation Parameters of Head and Neck Cancer Patients.Cancers (Basel). 2019 Sep 6;11(9):1324. doi: 10.3390/cancers11091324. Cancers (Basel). 2019. PMID: 31500214 Free PMC article.
-
Stimulating T Cells Against Cancer With Agonist Immunostimulatory Monoclonal Antibodies.Int Rev Cell Mol Biol. 2019;342:1-25. doi: 10.1016/bs.ircmb.2018.07.003. Epub 2018 Aug 20. Int Rev Cell Mol Biol. 2019. PMID: 30635089 Free PMC article. Review.
References
-
- Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. The Journal of experimental medicine. 2006;203(5):1259–71. Epub 2006/04/26. 10.1084/jem.20052494 - DOI - PMC - PubMed
-
- Gough MJ, Crittenden MR, Sarff M, Pang P, Seung SK, Vetto JT, et al. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J Immunother. 2010;33(8):798–809. Epub 2010/09/16. 10.1097/CJI.0b013e3181ee7095 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

