One-year water-ageing of calcium phosphate composite containing nano-silver and quaternary ammonium to inhibit biofilms
- PMID: 27281037
- PMCID: PMC5113087
- DOI: 10.1038/ijos.2016.13
One-year water-ageing of calcium phosphate composite containing nano-silver and quaternary ammonium to inhibit biofilms
Abstract
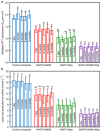
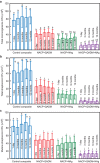
Dental composites are commonly used restorative materials; however, secondary caries due to biofilm acids remains a major problem. The objectives of this study were (1) to develop a composite containing quaternary ammonium dimethacrylate (QADM), nanoparticles of silver (NAg), and nanoparticles of amorphous calcium phosphate (NACP), and (2) to conduct the first investigation of the mechanical properties, biofilm response and acid production vs water-ageing time from 1 day to 12 months. A 4 × 5 design was utilized, with four composites (NACP-QADM composite, NACP-NAg composite, NACP-QADM-NAg composite, and a commercial control composite), and five water-ageing time periods (1 day, and 3, 6, 9, and 12 months). After each water-ageing period, the mechanical properties of the resins were measured in a three-point flexure, and antibacterial properties were tested via a dental plaque biofilm model using human saliva as an inoculum. After 12 months of water-ageing, NACP-QADM-NAg had a flexural strength and elastic modulus matching those of the commercial control (P>0.1). Incorporation of QADM or NAg into the NACP composite greatly reduced biofilm viability, metabolic activity and acid production. A composite containing both QADM and NAg possessed a stronger antibacterial capability than one with QADM or NAg alone (P<0.05). The anti-biofilm activity was maintained after 12 months of water-ageing and showed no significant decrease with increasing time (P>0.1). In conclusion, the NACP-QADM-NAg composite decreased biofilm viability and lactic acid production, while matching the load-bearing capability of a commercial composite. There was no decrease in its antibacterial properties after 1 year of water-ageing. The durable antibacterial and mechanical properties indicate that NACP-QADM-NAg composites may be useful in dental restorations to combat caries.
Figures


Similar articles
-
Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles.Dent Mater. 2012 May;28(5):561-72. doi: 10.1016/j.dental.2012.01.005. Epub 2012 Feb 2. Dent Mater. 2012. PMID: 22305716 Free PMC article.
-
Dental plaque microcosm biofilm behavior on calcium phosphate nanocomposite with quaternary ammonium.Dent Mater. 2012 Aug;28(8):853-62. doi: 10.1016/j.dental.2012.04.024. Epub 2012 May 10. Dent Mater. 2012. PMID: 22578992 Free PMC article.
-
Antibacterial nanocomposite with calcium phosphate and quaternary ammonium.J Dent Res. 2012 May;91(5):460-6. doi: 10.1177/0022034512440579. Epub 2012 Mar 8. J Dent Res. 2012. PMID: 22403412 Free PMC article.
-
Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries.Nanomedicine (Lond). 2015 Mar;10(4):627-41. doi: 10.2217/nnm.14.191. Nanomedicine (Lond). 2015. PMID: 25723095 Free PMC article. Review.
-
How we are assessing the developing antibacterial resin-based dental materials? A scoping review.J Dent. 2020 Aug;99:103369. doi: 10.1016/j.jdent.2020.103369. Epub 2020 May 7. J Dent. 2020. PMID: 32387506
Cited by
-
Nanotechnology in toothpaste: Fundamentals, trends, and safety.Heliyon. 2024 Jan 20;10(3):e24949. doi: 10.1016/j.heliyon.2024.e24949. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38317872 Free PMC article. Review.
-
Antibacterial Agents for Composite Resin Restorative Materials: Current Knowledge and Future Prospects.Cureus. 2024 Mar 29;16(3):e57212. doi: 10.7759/cureus.57212. eCollection 2024 Mar. Cureus. 2024. PMID: 38681374 Free PMC article. Review.
-
Developing a New Generation of Antimicrobial and Bioactive Dental Resins.J Dent Res. 2017 Jul;96(8):855-863. doi: 10.1177/0022034517709739. Epub 2017 May 22. J Dent Res. 2017. PMID: 28530844 Free PMC article. Review.
-
Novel Nanocomposite Inhibiting Caries at the Enamel Restoration Margins in an In Vitro Saliva-Derived Biofilm Secondary Caries Model.Int J Mol Sci. 2020 Sep 2;21(17):6369. doi: 10.3390/ijms21176369. Int J Mol Sci. 2020. PMID: 32887330 Free PMC article.
-
Applications of Silver Nanoparticles in Dentistry: Advances and Technological Innovation.Int J Mol Sci. 2021 Mar 2;22(5):2485. doi: 10.3390/ijms22052485. Int J Mol Sci. 2021. PMID: 33801230 Free PMC article. Review.
References
-
- Bagramian RA, Garcia-Godoy F, Volpe AR. The global increase in dental caries. A pending public health crisis. Am J Dent 2009; 22 (1): 3–8. - PubMed
-
- Bayne SC, Thompson JY, Swift EJ Jr et al. A characterization of first-generation flowable composites. J Am Dent Assoc 1998; 129 (5): 567–577. - PubMed
-
- Lim BS, Ferracane JL, Sakaguchi RL et al. Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dent Mater 2002; 18 (6): 436–444. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

