Efficacy of rabbit anti-thymocyte globulin for steroid-resistant acute rejection after liver transplantation
- PMID: 27281070
- PMCID: PMC4907648
- DOI: 10.1097/MD.0000000000003711
Efficacy of rabbit anti-thymocyte globulin for steroid-resistant acute rejection after liver transplantation
Erratum in
-
Erratum: Medicine, Volume 95, Issue 23: Erratum.Medicine (Baltimore). 2016 Jul 18;95(28):e0916. doi: 10.1097/01.md.0000489580.04709.16. eCollection 2016 Jul. Medicine (Baltimore). 2016. PMID: 31265603 Free PMC article.
Abstract
Acute cellular rejection after liver transplantation (LT) can be treated with steroid pulse therapy, but there is no ideal treatment for steroid-resistant acute rejection (SRAR). We aimed to determine the feasibility and potential complications of rabbit anti-thymocyte globulin (rATG) application to treat SRAR in liver transplant recipients. We retrospectively reviewed medical records of 429 recipients who underwent LT at Severance Hospital between January 2010 and March 2015. We compared clinical features and graft survival between patients with steroid-sensitive acute rejection (SSAR; n = 23) and SRAR (n = 11). We also analyzed complications and changes in laboratory findings after 2.5 mg/kg rATG treatment in patients with SRAR for 6 to 10 days. There were no significant differences in gender, age, model for end-stage liver disease score, Child-Turcotte-Pugh score, or original liver diseases between patients with SSAR and SRAR, although deceased donors were more frequently associated with the SRAR group (P = 0.004). All SRAR patients responded positively to rATG treatment; after treatment, the patients' median AST levels decreased from 138 to 63 IU/L, and their median ALT levels dropped from 327 to 70 IU/L 1 day after rATG treatment (P = 0.022 and 0.017, respectively). Median aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin levels significantly decreased 1 month post-treatment (P = 0.038, 0.004, and 0.041, respectively). Median survival after LT was 23 months, and median survival after rATG was 22 months in patients with SRAR. Adverse effects included hepatitis C virus (HCV) reactivation, fungemia, and cytomegalovirus (CMV) infection. Nine SRAR patients survived with healthy liver function, 1 died from a traffic accident during follow-up, and 1 died from graft-versus-host disease and fungemia. Administration of rATG is an effective therapeutic option for SRAR with acceptable complications in liver transplant recipients. However, the occurrence of HCV reactivation and CMV infection in LT patients should be monitored after rATG treatment in these patients.
Conflict of interest statement
The authors have no funding and conflicts of interest to disclose.
Figures


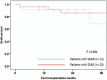
References
-
- Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2013 Annual Data Report: liver. Am J Transplant 2015; 15 Suppl 2:1–28. - PubMed
-
- Balderramo D, Navasa M, Cardenas A. Current management of biliary complications after liver transplantation: emphasis on endoscopic therapy. Gastroenterologia Hepatologia 2011; 34:107–115. - PubMed
-
- Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant 2009; 9:746–757. - PubMed
-
- Andreu H, Rimola A, Bruguera M, et al. Acute cellular rejection in liver transplant recipients under cyclosporine immunosuppression: predictive factors of response to antirejection therapy. Transplantation 2002; 73:1936–1943. - PubMed
-
- Wu L, Tam N, Deng R, et al. Steroid-resistant acute rejection after cadaveric liver transplantation: Experience from one single center. Clinics Res Hepatol Gastroenterol 2014; 38:592–597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

