National trends in off-label use of atypical antipsychotics in children and adolescents in the United States
- PMID: 27281081
- PMCID: PMC4907659
- DOI: 10.1097/MD.0000000000003784
National trends in off-label use of atypical antipsychotics in children and adolescents in the United States
Erratum in
-
Erratum: Medicine, Volume 95, Issue 23: Erratum.Medicine (Baltimore). 2016 Jul 18;95(28):e0916. doi: 10.1097/01.md.0000489580.04709.16. eCollection 2016 Jul. Medicine (Baltimore). 2016. PMID: 31265603 Free PMC article.
Abstract
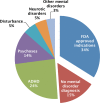
The objectives of the study were as follows: to examine the national trend of pediatric atypical antipsychotic (AAP) use in the United States; to identify primary mental disorders associated with AAPs; to estimate the strength of independent associations between patient/provider characteristics and AAP use. Data are from the National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey. First, average AAP prescription rates among 4 and 18-year-old patients between 1993 and 2010 were estimated. Second, data from 2007 to 2010 were combined and analyzed to identify primary mental disorders related to AAP prescription. Third, a multivariate logistic regression model was developed having the presence of AAP prescription as the dependent variable and patient/provider characteristics as explanatory variables. Adjusted odds ratios (AORs) with associated 95% confidence intervals (CIs) were estimated. Outpatient visits including an AAP prescription among 4 to 18-year-old patients significantly increased between 1993 and 2010 in the United States, and over 65% of those visits did not have diagnoses for US Food and Drug Administration-approved AAP indications. During 2007 to 2010, the most common mental disorder was attention-deficit hyperactivity disorder, accounting for 24% of total pediatric AAP visits. Among visits with attention-deficit hyperactivity disorder diagnosis, those with Medicaid as payer (AOR 1.66, 95% CI 1.01-2.75), comorbid mental disorders (e.g., psychoses AOR 3.34, 95% CI 1.35-8.26), and multiple prescriptions (4 or more prescriptions AOR 4.48, 95% CI 2.08-9.64) were more likely to have an AAP prescription. The off-label use of AAPs in children and adolescents is prevalent in the United States. Our study raises questions about the potential misuse of AAPs in the population.
Conflict of interest statement
The authors declare that there are no conflicts of interest to disclose.
Figures
References
-
- Pieters T, Majerus B. The introduction of chlorpromazine in Belgium and the Netherlands (1951-1968); tango between old and new treatment features. Stud History Philos Biol Biomed Sci 2011; 42:443–452. - PubMed
-
- Seida JC, Schouten JR, Boylan K, et al. Antipsychotics for children and young adults: a comparative effectiveness review. Pediatrics 2012; 129:e771–784. - PubMed
-
- Scheltema Beduin A, de Haan L. Off-label second generation antipsychotics for impulse regulation disorders: a review. Psychopharmacol Bull 2010; 43:45–81. - PubMed
-
- Pathak P, West D, Martin BC, et al. Evidence-based use of second-generation antipsychotics in a state Medicaid pediatric population, 2001-2005. Psychiatric Serv 2010; 61:123–129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous