Targeting the Microglial Signaling Pathways: New Insights in the Modulation of Neuropathic Pain
- PMID: 27281131
- PMCID: PMC5427777
- DOI: 10.2174/0929867323666160607120124
Targeting the Microglial Signaling Pathways: New Insights in the Modulation of Neuropathic Pain
Abstract
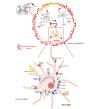
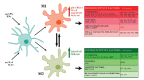
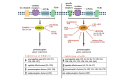
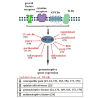
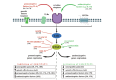
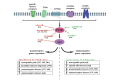
The microglia, once thought only to be supporting cells of the central nervous system (CNS), are now recognized to play essential roles in many pathologies. Many studies within the last decades indicated that the neuro-immune interaction underlies the generation and maintenance of neuropathic pain. Through a large number of receptors and signaling pathways, the microglial cells communicate with neurons, astrocytes and other cells, including those of the immune system. A disturbance or loss of CNS homeostasis causes rapid responses of the microglia, which undergo a multistage activation process. The activated microglia change their cell shapes and gene expression profiles, which induce proliferation, migration, and the production of pro- or antinociceptive factors. The cells release a large number of mediators that can act in a manner detrimental or beneficial to the surrounding cells and can indirectly alter the nociceptive signals. This review discusses the most important microglial intracellular signaling cascades (MAPKs, NF-kB, JAK/STAT, PI3K/Akt) that are essential for neuropathic pain development and maintenance. Our objective was to identify new molecular targets that may result in the development of powerful tools to control the signaling associated with neuropathic pain.
Figures
References
-
- Merskey H., Bogduk N. Classification of Chronic Pain. Seattle: IASP Press; 1994.
-
- Coyle D.E. Partial peripheral nerve injury leads to activation of astroglia and microglia which parallels the development of allodynic behavior. Glia. 1998;23:75–83. - PubMed
-
- Dworkin R.H., O’Connor A.B., Audette J., Baron R., Gourlay G.K., Haanpää M.L., Kent J.L., Krane E.J., Lebel A.A., Levy R.M., Mackey S.C., Mayer J., Miaskowski C., Raja S.N., Rice A.S., Schmader K.E., Stacey B., Stanos S., Treede R.D., Turk D.C., Walco G.A., Wells C.D. Recommendations for the pharma-cological management of neuropathic pain: an overview and literature update. Mayo Clin. Proc. 2010;85:S3–S14. - PMC - PubMed
-
- Kalso E., Allan L., Dellemijn P.L., Faura C.C., Ilias W.K., Jensen T.S., Perrot S., Plaghki L.H., Zenz M. Recommendations for using opioids in chronic non-cancer pain. Eur. J. Pain. 2003;7:381–386. - PubMed
-
- Kalso E., Edwards J.E., Moore R.A., McQuay H.J. Opioids in chronic non-cancer pain: Systematic review of efficacy and safety. Pain. 2004;112:372–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

