Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain
- PMID: 27281198
- PMCID: PMC4919188
- DOI: 10.1038/nature17976
Selective spider toxins reveal a role for the Nav1.1 channel in mechanical pain
Abstract
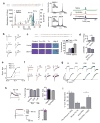
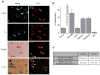
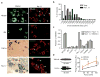
Voltage-gated sodium (Nav) channels initiate action potentials in most neurons, including primary afferent nerve fibres of the pain pathway. Local anaesthetics block pain through non-specific actions at all Nav channels, but the discovery of selective modulators would facilitate the analysis of individual subtypes of these channels and their contributions to chemical, mechanical, or thermal pain. Here we identify and characterize spider (Heteroscodra maculata) toxins that selectively activate the Nav1.1 subtype, the role of which in nociception and pain has not been elucidated. We use these probes to show that Nav1.1-expressing fibres are modality-specific nociceptors: their activation elicits robust pain behaviours without neurogenic inflammation and produces profound hypersensitivity to mechanical, but not thermal, stimuli. In the gut, high-threshold mechanosensitive fibres also express Nav1.1 and show enhanced toxin sensitivity in a mouse model of irritable bowel syndrome. Together, these findings establish an unexpected role for Nav1.1 channels in regulating the excitability of sensory nerve fibres that mediate mechanical pain.
Conflict of interest statement
The authors declare competing financial interests: details are available in the online version of the paper.
Figures







References
-
- Dib-Hajj SD, Yang Y, Black JA, Waxman SG. The NaV1.7 sodium channel: from molecule to man. Nat Rev Neurosci. 2013;14:49–62. - PubMed
-
- Fertleman CR, et al. SCN9A mutations in paroxysmal extreme pain disorder: allelic variants underlie distinct channel defects and phenotypes. Neuron. 2006;52:767–774. - PubMed
-
- Hoeijmakers JG, Faber CG, Merkies IS, Waxman SG. Painful peripheral neuropathy and sodium channel mutations. Neurosci Lett. 2015;596:51–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F30 DE023476/DE/NIDCR NIH HHS/United States
- R01NS040538/NS/NINDS NIH HHS/United States
- R01 NS014627/NS/NINDS NIH HHS/United States
- F31NS084646/NS/NINDS NIH HHS/United States
- R01 NS081115/NS/NINDS NIH HHS/United States
- R01DA29204/DA/NIDA NIH HHS/United States
- F31 NS084646/NS/NINDS NIH HHS/United States
- R01 NS070711/NS/NINDS NIH HHS/United States
- R37 NS065071/NS/NINDS NIH HHS/United States
- T32 HL007731/HL/NHLBI NIH HHS/United States
- F32NS081907/NS/NINDS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- R01 DA029204/DA/NIDA NIH HHS/United States
- F30DE023476/DE/NIDCR NIH HHS/United States
- R37 NS014627/NS/NINDS NIH HHS/United States
- R01NS091352/NS/NINDS NIH HHS/United States
- F32 NS081907/NS/NINDS NIH HHS/United States
- R01 NS040538/NS/NINDS NIH HHS/United States
- R37NS065071/NS/NINDS NIH HHS/United States
- R01NS081115/NS/NINDS NIH HHS/United States
- R01 NS091352/NS/NINDS NIH HHS/United States
- R37NS014627/NS/NINDS NIH HHS/United States
- R01NS070711/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

