Prevention of pancreatic cancer in a hamster model by cAMP decrease
- PMID: 27281617
- PMCID: PMC5190108
- DOI: 10.18632/oncotarget.9790
Prevention of pancreatic cancer in a hamster model by cAMP decrease
Abstract
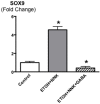
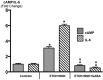
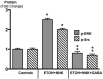
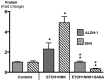
Smoking and alcoholism are risk factors for the development of pancreatitis-associated pancreatic ductal adenocarcinoma (PDAC). We have previously shown that these cancers overexpressed stress neurotransmitters and cyclic adenosine monophosphate (cAMP) while the inhibitory neurotransmitter γ-aminobutyric acid (GABA) was suppressed. Using a hamster model, the current study has tested the hypothesis that cAMP decrease by GABA supplementation in the drinking water prevents the development of pancreatitis-associated PDAC. Our data reveal strong preventive effects of GABA supplementation on the development of PDAC and pancreatic intraductal neoplasia (PanIN). ELISA assays and immunohistochemistry revealed significant decreases in the levels of cAMP and interleukin 6 accompanied by reductions in the expression of several cancer stem cell markers and phosphorylated signaling proteins, which stimulate cell proliferation, and migration in pancreatic exocrine cells of GABA treated animals. We conclude that cAMP decrease by GABA supplementation inhibits multiple cancer stimulating pathways in cancer stem cells, differentiated cancer cells and the immune system, identifying this approach as promising novel tool for the prevention of PDAC in individuals with a history of smoking and alcoholism.
Keywords: cancer stem cell signaling; cyclic adenosine monophosphate; pancreatic cancer; prevention; γ-aminobutyric acid.
Conflict of interest statement
None to declare.
Figures






Similar articles
-
Prevention of pancreatic cancer by the beta-blocker propranolol.Anticancer Drugs. 2009 Jul;20(6):477-82. doi: 10.1097/CAD.0b013e32832bd1e3. Anticancer Drugs. 2009. PMID: 19387337 Free PMC article.
-
Nicotinic receptor-associated modulation of stimulatory and inhibitory neurotransmitters in NNK-induced adenocarcinoma of the lungs and pancreas.J Pathol. 2009 Aug;218(4):437-45. doi: 10.1002/path.2542. J Pathol. 2009. PMID: 19274673 Free PMC article.
-
Nicotine stimulates pancreatic cancer xenografts by systemic increase in stress neurotransmitters and suppression of the inhibitory neurotransmitter gamma-aminobutyric acid.Carcinogenesis. 2009 Mar;30(3):506-11. doi: 10.1093/carcin/bgp010. Epub 2009 Jan 8. Carcinogenesis. 2009. PMID: 19131543 Free PMC article.
-
Regulatory Role of G Protein-coupled Receptors in Pancreatic Cancer Development and Progression.Curr Med Chem. 2018;25(22):2566-2575. doi: 10.2174/0929867324666170303121708. Curr Med Chem. 2018. PMID: 28260499 Review.
-
Acinar-to-Ductal Metaplasia (ADM): On the Road to Pancreatic Intraepithelial Neoplasia (PanIN) and Pancreatic Cancer.Int J Mol Sci. 2023 Jun 9;24(12):9946. doi: 10.3390/ijms24129946. Int J Mol Sci. 2023. PMID: 37373094 Free PMC article. Review.
Cited by
-
Investigation of the potential role of TGR5 in pancreatic cancer by a comprehensive molecular experiments and the liquid chromatography mass spectrometry (LC-MS) based metabolomics.Discov Oncol. 2022 Jun 11;13(1):46. doi: 10.1007/s12672-022-00504-2. Discov Oncol. 2022. PMID: 35689739 Free PMC article.
-
Crosstalk of nervous and immune systems in pancreatic cancer.Front Cell Dev Biol. 2023 Nov 30;11:1309738. doi: 10.3389/fcell.2023.1309738. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38099290 Free PMC article. Review.
-
The MAZ transcription factor is a downstream target of the oncoprotein Cyr61/CCN1 and promotes pancreatic cancer cell invasion via CRAF-ERK signaling.J Biol Chem. 2018 Mar 23;293(12):4334-4349. doi: 10.1074/jbc.RA117.000333. Epub 2018 Feb 6. J Biol Chem. 2018. PMID: 29414775 Free PMC article.
-
Pathological Changes in Pancreatic Carcinogenesis: A Review.Cancers (Basel). 2021 Feb 8;13(4):686. doi: 10.3390/cancers13040686. Cancers (Basel). 2021. PMID: 33567676 Free PMC article. Review.
-
CD38 affects the biological behavior and energy metabolism of nasopharyngeal carcinoma cells.Int J Oncol. 2019 Feb;54(2):585-599. doi: 10.3892/ijo.2018.4651. Epub 2018 Dec 3. Int J Oncol. 2019. PMID: 30535454 Free PMC article.
References
-
- Yeo TP. Demographics, Epidemiology, and Inheritance of Pancreatic Ductal Adenocarcinoma. Seminars in oncology. 2015;42:8–18. - PubMed
-
- Malvezzi M, Bertuccio P, Rosso T, Rota M, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2015: does lung cancer have the highest death rate in EU women? Ann Oncol. 2015;26:779–786. - PubMed
-
- Schuller HM, Jorquera R, Reichert A, Castonguay A. Transplacental induction of pancreas tumors in hamsters by ethanol and the tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 1993;53:2498–2501. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

