Immunoproteomic identification of MbovP579, a promising diagnostic biomarker for serological detection of Mycoplasma bovis infection
- PMID: 27281618
- PMCID: PMC5129939
- DOI: 10.18632/oncotarget.9799
Immunoproteomic identification of MbovP579, a promising diagnostic biomarker for serological detection of Mycoplasma bovis infection
Abstract
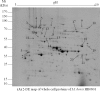
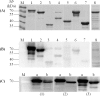
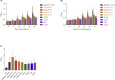
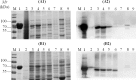
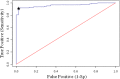
A lack of knowledge regarding the antigenic properties of Mycoplasma bovis proteins prevents the effective control of bovine infections using immunological approaches. In this study, we detected and characterized a specific and sensitive M. bovis diagnostic biomarker. After M. bovis total proteins and membrane fractions were separated with two dimensional gel electrophoresis, proteins reacting with antiserawere detected using MALDI-TOF MS. Thirty-nine proteins were identified, 32 of which were previously unreported. Among them, immunoinformatics predicted eight antigens, encoded by Mbov_0106, 0116, 0126, 0212, 0275, 0579, 0739, and 0789, to have high immunological value. These genes were expressed in E. coli after mutagenesis of UGA to UGG using overlap extension PCR. A lipoprotein, MbovP579, encoded by a functionally unknown gene, was a sensitive and specific antigen for detection of antibodies in sera from both M. bovis-infected and vaccinated cattle. The specificity of MbovP579 was confirmed by its lack of cross-reactivity with other mycoplasmas, including Mycoplasma agalactiae. An iELISA based on rMbovP579 detected seroconversion 7 days post-infection (dpi). The ELISA had sensitivity of 90.2% (95% CI: 83.7%, 94.3%) and a specificity of 97.8% (95% CI: 88.7%, 99.6%) with clinical samples. Additional comparative studies showed that both diagnostic and analytic sensitivities of the ELISA were higher than those of a commercially available kit (p<0.01). We have thus detected and characterized the novel antigen, MbovP579, and established an rMbovP579-based ELISA as a highly sensitive and specific method for the early diagnosis of M. bovis infection.
Keywords: ELISA; Immune response; Immunity; Immunology and Microbiology Section; Mycoplasma bovis; diagnostic biomarker; immunoinformatics; immunoproteomics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Identification of novel immunogenic proteins from Mycoplasma bovis and establishment of an indirect ELISA based on recombinant E1 beta subunit of the pyruvate dehydrogenase complex.PLoS One. 2014 Feb 10;9(2):e88328. doi: 10.1371/journal.pone.0088328. eCollection 2014. PLoS One. 2014. PMID: 24520369 Free PMC article.
-
Development of a recombinant protein-based enzyme-linked immunosorbent assay for diagnosis of Mycoplasma bovis infection in cattle.Clin Vaccine Immunol. 2014 Feb;21(2):196-202. doi: 10.1128/CVI.00670-13. Epub 2013 Dec 11. Clin Vaccine Immunol. 2014. PMID: 24334686 Free PMC article.
-
Genetic and antigenic characterization of the surface lipoprotein P48 of Mycoplasma bovis.Vet Microbiol. 2005 Aug 30;109(3-4):201-9. doi: 10.1016/j.vetmic.2005.05.007. Vet Microbiol. 2005. PMID: 15985342
-
Proteomics analysis and its role in elucidation of functionally significant proteins in Mycoplasma bovis.Microb Pathog. 2017 Oct;111:50-59. doi: 10.1016/j.micpath.2017.08.024. Epub 2017 Aug 18. Microb Pathog. 2017. PMID: 28826762 Review.
-
Comparison of various diagnostic methods for the detection of Mycoplasma bovis.Rev Sci Tech. 1993 Jun;12(2):571-80. doi: 10.20506/rst.12.2.701. Rev Sci Tech. 1993. PMID: 8400393 Review.
Cited by
-
MbovP0725, a secreted serine/threonine phosphatase, inhibits the host inflammatory response and affects metabolism in Mycoplasma bovis.mSystems. 2024 Apr 16;9(4):e0089123. doi: 10.1128/msystems.00891-23. Epub 2024 Mar 5. mSystems. 2024. PMID: 38440990 Free PMC article.
-
Clinicopathological and Sero-Molecular Detection of Mycoplasma capricolum subsp. capripneumoniae in Goats in Southern Areas of Pakistan.Vet Med Int. 2022 Oct 3;2022:9508810. doi: 10.1155/2022/9508810. eCollection 2022. Vet Med Int. 2022. PMID: 36226029 Free PMC article.
-
Proteomics identification and characterization of MbovP730 as a potential DIVA antigen of Mycoplasma bovis.Oncotarget. 2017 Nov 2;9(47):28322-28336. doi: 10.18632/oncotarget.22265. eCollection 2018 Jun 19. Oncotarget. 2017. PMID: 29983863 Free PMC article.
-
Mycoplasmas as Host Pantropic and Specific Pathogens: Clinical Implications, Gene Transfer, Virulence Factors, and Future Perspectives.Front Cell Infect Microbiol. 2022 May 13;12:855731. doi: 10.3389/fcimb.2022.855731. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35646746 Free PMC article. Review.
-
Prevalence and Whole Genome Sequence Analysis of Mycoplasma bovis Isolates From Bulk Tank Milk of Dairy Farms in Tennessee, USA.J Vet Intern Med. 2025 Jul-Aug;39(4):e70164. doi: 10.1111/jvim.70164. J Vet Intern Med. 2025. PMID: 40525812 Free PMC article.
References
-
- Nicholas RAJ, Ayling RD. Mycoplasma bovis: disease, diagnosis, and control. Res Vet Sci. 2003;74:105–112. - PubMed
-
- Mustafa R, Qi J, Ba X, Chen Y, Hu C, Liu X, Tu L, Peng Q, Chen H, Guo A. In vitro Quinolones Susceptibility Analysis of Chinese Mycoplasma bovis Isolates and their Phylogenetic Scenarios based upon QRDRs of DNA Topoisomerases Revealing a Unique Transition in ParC. Pak Vet J. 2013;33:364–369.
-
- Zhang R, Han X, Chen Y, Mustafa R, Qi J, Chen X, Hu C, Chen H, Guo A. Attenuated Mycoplasma bovis strains provide protection against virulent infection in calves. Vaccine. 2014;32:3107–3114. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

