A physiological and behavioral system for hearing restoration with cochlear implants
- PMID: 27281743
- PMCID: PMC4995281
- DOI: 10.1152/jn.00048.2016
A physiological and behavioral system for hearing restoration with cochlear implants
Abstract
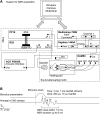
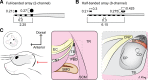
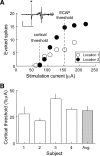
Cochlear implants are neuroprosthetic devices that provide hearing to deaf patients, although outcomes are highly variable even with prolonged training and use. The central auditory system must process cochlear implant signals, but it is unclear how neural circuits adapt-or fail to adapt-to such inputs. The knowledge of these mechanisms is required for development of next-generation neuroprosthetics that interface with existing neural circuits and enable synaptic plasticity to improve perceptual outcomes. Here, we describe a new system for cochlear implant insertion, stimulation, and behavioral training in rats. Animals were first ensured to have significant hearing loss via physiological and behavioral criteria. We developed a surgical approach for multichannel (2- or 8-channel) array insertion, comparable with implantation procedures and depth in humans. Peripheral and cortical responses to stimulation were used to program the implant objectively. Animals fitted with implants learned to use them for an auditory-dependent task that assesses frequency detection and recognition in a background of environmentally and self-generated noise and ceased responding appropriately to sounds when the implant was temporarily inactivated. This physiologically calibrated and behaviorally validated system provides a powerful opportunity to study the neural basis of neuroprosthetic device use and plasticity.
Keywords: auditory cortex; behavior; cochlear implants; deafness; rats.
Copyright © 2016 the American Physiological Society.
Figures






References
-
- NIDCD Fact Sheet, Cochlear Implants. NIH Publication No. 11-4798 U.S. Department of Health and Human Services, National Institutes of Health, National Institute on Deafness and Other Communication Disorders, NIDCD Information Clearinghouse, Bethesda, MD, 2011.
-
- Agterberg MJ, Versnel H. Behavioral responses of deafened guinea pigs to intracochlear electrical stimulation: a new rapid psychophysical procedure. Hear Res 313: 67–74, 2014. - PubMed
-
- Agterberg MJ, Versnel H, de Groot JC, van den Broek M, Klis SF. Chronic electrical stimulation does not prevent spiral ganglion cell degeneration in deafened guinea pigs. Hear Res 269: 169–179, 2010. - PubMed
-
- Beitel RE, Vollmer M, Snyder RL, Schreiner CE, Leake PA. Behavioral and neurophysiological thresholds for electrical cochlear stimulation in the deaf cat. Audiol Neurootol 5: 31–38, 2000. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

