Octopamine stabilizes conduction reliability of an unmyelinated axon during hypoxic stress
- PMID: 27281750
- PMCID: PMC5009204
- DOI: 10.1152/jn.00354.2016
Octopamine stabilizes conduction reliability of an unmyelinated axon during hypoxic stress
Abstract
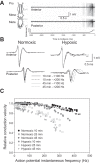
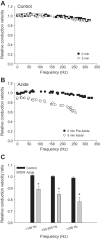
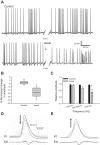
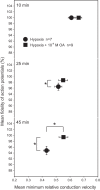
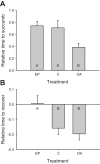
Mechanisms that could mitigate the effects of hypoxia on neuronal signaling are incompletely understood. We show that axonal performance of a locust visual interneuron varied depending on oxygen availability. To induce hypoxia, tracheae supplying the thoracic nervous system were surgically lesioned and action potentials in the axon of the descending contralateral movement detector (DCMD) neuron passing through this region were monitored extracellularly. The conduction velocity and fidelity of action potentials decreased throughout a 45-min experiment in hypoxic preparations, whereas conduction reliability remained constant when the tracheae were left intact. The reduction in conduction velocity was exacerbated for action potentials firing at high instantaneous frequencies. Bath application of octopamine mitigated the loss of conduction velocity and fidelity. Action potential conduction was more vulnerable in portions of the axon passing through the mesothoracic ganglion than in the connectives between ganglia, indicating that hypoxic modulation of the extracellular environment of the neuropil has an important role to play. In intact locusts, octopamine and its antagonist, epinastine, had effects on the entry to, and recovery from, anoxic coma consistent with octopamine increasing overall neural performance during hypoxia. These effects could have functional relevance for the animal during periods of environmental or activity-induced hypoxia.
Keywords: DCMD; action potential; conduction velocity; locust; sodium azide.
Copyright © 2016 the American Physiological Society.
Figures


References
-
- Ayali A, Pener MP. Density-dependent phase polymorphism affects response to adipokinetic hormone in Locusta. Comp Biochem Physiol A 101: 549–552, 1992.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

