RNA splicing factors as oncoproteins and tumour suppressors
- PMID: 27282250
- PMCID: PMC5094465
- DOI: 10.1038/nrc.2016.51
RNA splicing factors as oncoproteins and tumour suppressors
Abstract
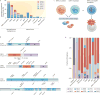
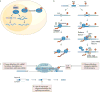
The recent genomic characterization of cancers has revealed recurrent somatic point mutations and copy number changes affecting genes encoding RNA splicing factors. Initial studies of these 'spliceosomal mutations' suggest that the proteins bearing these mutations exhibit altered splice site and/or exon recognition preferences relative to their wild-type counterparts, resulting in cancer-specific mis-splicing. Such changes in the splicing machinery may create novel vulnerabilities in cancer cells that can be therapeutically exploited using compounds that can influence the splicing process. Further studies to dissect the biochemical, genomic and biological effects of spliceosomal mutations are crucial for the development of cancer therapies targeted at these mutations.
Conflict of interest statement
statement The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

