Presynaptic Spike Timing-Dependent Long-Term Depression in the Mouse Hippocampus
- PMID: 27282393
- PMCID: PMC4961031
- DOI: 10.1093/cercor/bhw172
Presynaptic Spike Timing-Dependent Long-Term Depression in the Mouse Hippocampus
Abstract
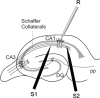
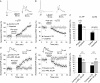
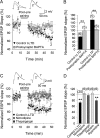
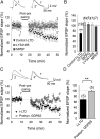
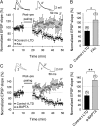
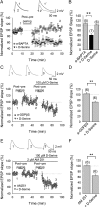
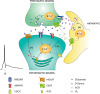
Spike timing-dependent plasticity (STDP) is a Hebbian learning rule important for synaptic refinement during development and for learning and memory in the adult. Given the importance of the hippocampus in memory, surprisingly little is known about the mechanisms and functions of hippocampal STDP. In the present work, we investigated the requirements for induction of hippocampal spike timing-dependent long-term potentiation (t-LTP) and spike timing-dependent long-term depression (t-LTD) and the mechanisms of these 2 forms of plasticity at CA3-CA1 synapses in young (P12-P18) mouse hippocampus. We found that both t-LTP and t-LTD can be induced at hippocampal CA3-CA1 synapses by pairing presynaptic activity with single postsynaptic action potentials at low stimulation frequency (0.2 Hz). Both t-LTP and t-LTD require NMDA-type glutamate receptors for their induction, but the location and properties of these receptors are different: While t-LTP requires postsynaptic ionotropic NMDA receptor function, t-LTD does not, and whereas t-LTP is blocked by antagonists at GluN2A and GluN2B subunit-containing NMDA receptors, t-LTD is blocked by GluN2C or GluN2D subunit-preferring NMDA receptor antagonists. Both t-LTP and t-LTD require postsynaptic Ca(2+) for their induction. Induction of t-LTD also requires metabotropic glutamate receptor activation, phospholipase C activation, postsynaptic IP3 receptor-mediated Ca(2+) release from internal stores, postsynaptic endocannabinoid (eCB) synthesis, activation of CB1 receptors and astrocytic signaling, possibly via release of the gliotransmitter d-serine. We furthermore found that presynaptic calcineurin is required for t-LTD induction. t-LTD is expressed presynaptically as indicated by fluctuation analysis, paired-pulse ratio, and rate of use-dependent depression of postsynaptic NMDA receptor currents by MK801. The results show that CA3-CA1 synapses display both NMDA receptor-dependent t-LTP and t-LTD during development and identify a presynaptic form of hippocampal t-LTD similar to that previously described at neocortical synapses during development.
Keywords: NMDA receptor; hippocampus; spike timing-dependent plasticity; t-LTD; t-LTP.
© The Author 2016. Published by Oxford University Press.
Figures



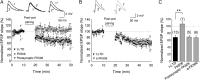

References
-
- Alger BE. 2002. Retrograde signalling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 68:247–286. - PubMed
-
- Anwyl R. 1999. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Rev. 29:83–120. - PubMed
-
- Auberson YP, Allegier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 2002. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather 1A/2B receptor composition. Bioorg Med Chem Lett. 12:1099–1102. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

