Multisensory Processes: A Balancing Act across the Lifespan
- PMID: 27282408
- PMCID: PMC4967384
- DOI: 10.1016/j.tins.2016.05.003
Multisensory Processes: A Balancing Act across the Lifespan
Abstract
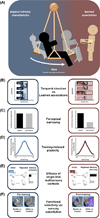
Multisensory processes are fundamental in scaffolding perception, cognition, learning, and behavior. How and when stimuli from different sensory modalities are integrated rather than treated as separate entities is poorly understood. We review how the relative reliance on stimulus characteristics versus learned associations dynamically shapes multisensory processes. We illustrate the dynamism in multisensory function across two timescales: one long term that operates across the lifespan and one short term that operates during the learning of new multisensory relations. In addition, we highlight the importance of task contingencies. We conclude that these highly dynamic multisensory processes, based on the relative weighting of stimulus characteristics and learned associations, provide both stability and flexibility to brain functions over a wide range of temporal scales.
Keywords: aging.; cross-modal; development; learning; multisensory; plasticity.
Copyright © 2016 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Sumby WH, Pollack I. Visual contribution to speech intelligibility in noise. J. Acoust. Soc. Am. 1954;26:212–215.
-
- Bavelier D, Neville HJ. Cross-modal plasticity: where and how? Nat. Rev. Neurosci. 2002;3:443–452. - PubMed
-
- Stein BE, editor. The New Handbook of Multisensory Processes. MIT Press; 2012.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

