In Vivo Tumor Angiogenesis Imaging Using Peptide-Based Near-Infrared Fluorescent Probes
- PMID: 27283419
- PMCID: PMC7469915
- DOI: 10.1007/978-1-4939-3721-9_8
In Vivo Tumor Angiogenesis Imaging Using Peptide-Based Near-Infrared Fluorescent Probes
Abstract
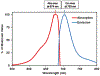
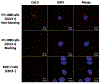
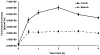
Near-infrared fluorescence (NIRF) imaging is an emerging imaging technique for studying diseases at the molecular level. Optical imaging with a near-infrared emitting fluorophore for targeting tumor angiogenesis offers a noninvasive method for early tumor detection and efficient monitoring of tumor response to anti-angiogenesis therapy. CD13 receptor, a zinc-dependent membrane-bound ectopeptidase, plays important roles in regulating tumor angiogenesis and the growth of new blood vessels. In this chapter, we use CD13 receptor as an example to demonstrate how to construct CD13-specific NGR-containing peptides via bioorthogonal click chemistry for visualizing and quantifying the CD13 receptor expression in vivo by means of NIRF optical imaging.
Keywords: Cancer; Molecular imaging; Near-infrared fluorescence imaging; Peptide-based probes; Tumor angiogenesis.
Figures


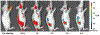


Similar articles
-
In vivo NIRF imaging-guided delivery of a novel NGR-VEGI fusion protein for targeting tumor vasculature.Amino Acids. 2014 Dec;46(12):2721-32. doi: 10.1007/s00726-014-1828-6. Epub 2014 Sep 3. Amino Acids. 2014. PMID: 25182731
-
Near-infrared fluorescence imaging of CD13 receptor expression using a novel Cy5.5-labeled dimeric NGR peptide.Amino Acids. 2014 Jun;46(6):1547-56. doi: 10.1007/s00726-014-1727-x. Epub 2014 Mar 21. Amino Acids. 2014. PMID: 24652439
-
MicroPET imaging of CD13 expression using a (64)Cu-labeled dimeric NGR peptide based on sarcophagine cage.Mol Pharm. 2014 Nov 3;11(11):3938-46. doi: 10.1021/mp500354x. Epub 2014 Jul 30. Mol Pharm. 2014. PMID: 25054774
-
Molecular Imaging of Aminopeptidase N in Cancer and Angiogenesis.Contrast Media Mol Imaging. 2018 Jun 25;2018:5315172. doi: 10.1155/2018/5315172. eCollection 2018. Contrast Media Mol Imaging. 2018. PMID: 30046296 Free PMC article. Review.
-
Molecular imaging based on metabolic glycoengineering and bioorthogonal click chemistry.Biomaterials. 2017 Jul;132:28-36. doi: 10.1016/j.biomaterials.2017.04.003. Epub 2017 Apr 4. Biomaterials. 2017. PMID: 28399460 Review.
Cited by
-
Early evaluation of anti-angiogenic effects with gadolinium(III) labeled APN/CD13 specific binding peptides magnetic resonance imaging.Sci Rep. 2025 Jul 1;15(1):21269. doi: 10.1038/s41598-025-05905-1. Sci Rep. 2025. PMID: 40596286 Free PMC article.
References
-
- Weissleder R, Tung CH, Mahmood U, Bogdanov A Jr (1999) In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol 17(4):375–378 - PubMed
-
- Tung CH (2004) Fluorescent peptide probes for in vivo diagnostic imaging. Biopolymers 76(5):391–403 - PubMed
-
- Chen X, Conti PS, Moats RA (2004) In vivo near-infrared fluorescence imaging of integrin alphavbeta3 in brain tumor xenografts. Cancer Res 64(21):8009–8014 - PubMed
-
- Chen K, Yap LP, Park R, Hui X, Wu K, Fan D, Chen X, Conti PS (20l2) A Cy5.5-labeled phage-displayed peptide probe for near-infrared fluorescence imaging of tumor vasculature in living mice. Amino Acids 42(4):1329–1337 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

