Current and Emerging Therapies for Lupus Nephritis
- PMID: 27283496
- PMCID: PMC5042683
- DOI: 10.1681/ASN.2016040415
Current and Emerging Therapies for Lupus Nephritis
Abstract
The introduction of corticosteroids and later, cyclophosphamide dramatically improved survival in patients with proliferative lupus nephritis, and combined administration of these agents became the standard-of-care treatment for this disease. However, treatment failures were still common and the rate of progression to ESRD remained unacceptably high. Additionally, treatment was associated with significant morbidity. Therefore, as patient survival improved, the goals for advancing lupus nephritis treatment shifted to identifying therapies that could improve long-term renal outcomes and minimize treatment-related toxicity. Unfortunately, progress has been slow and the current approaches to the management of lupus nephritis continue to rely on high-dose corticosteroids plus a broad-spectrum immunosuppressive agent. Over the past decade, an improved understanding of lupus nephritis pathogenesis fueled several clinical trials of novel drugs, but none have been found to be superior to the combination of a cytotoxic agent and corticosteroids. Despite these trial failures, efforts to translate mechanistic advances into new treatment approaches continue. In this review, we discuss current therapeutic strategies for lupus nephritis, briefly review recent advances in understanding the pathogenesis of this disease, and describe emerging approaches developed on the basis of these advances that promise to improve upon the standard-of-care lupus nephritis treatments.
Keywords: clinical trial; glomerular disease; immunosuppression; lupus nephritis; systemic lupus erythematosus.
Copyright © 2016 by the American Society of Nephrology.
Figures

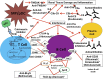
References
-
- Heller BI, Jacobson WE, Hammarsten JF. Effects of Cortisone in Glomerulonephritis and the Nephropathy of Disseminated Lupus Erythematosus. Am J Med 10: 520, 1951 - PubMed
-
- Cameron JS: Lupus nephritis. J Am Soc Nephrol 10: 413–424, 1999 - PubMed
-
- Pollak VE, Pirani CL, Schwartz FD: The natural history of the renal manifestations of systemic lupus erythematosus. 1964. J Am Soc Nephrol 8: 1189–1198; discussion 1189–1195, 1997 - PubMed
-
- Costenbader KH, Desai A, Alarcón GS, Hiraki LT, Shaykevich T, Brookhart MA, Massarotti E, Lu B, Solomon DH, Winkelmayer WC: Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum 63: 1681–1688, 2011 - PMC - PubMed
-
- Austin HA 3rd, Klippel JH, Balow JE, le Riche NG, Steinberg AD, Plotz PH, Decker JL: Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med 314: 614–619, 1986 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

