Quercetin solubilisation in bile salts: A comparison with sodium dodecyl sulphate
- PMID: 27283643
- PMCID: PMC4911888
- DOI: 10.1016/j.foodchem.2016.05.034
Quercetin solubilisation in bile salts: A comparison with sodium dodecyl sulphate
Abstract
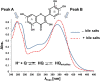
To understand the bioaccessibility of the flavonoid quercetin we studied its interaction with bile salt micelles. The environmental sensitivity of quercetin's UV-visible absorption spectrum gave information about quercetin partitioning. Two quercetin absorption peaks gave complementary information: Peak A (240-280nm) on the intermicellar phase and Peak B (340-440nm) on the micellar phase. Thus, by altering pH, we showed that only non-ionised quercetin partitions into micelles. We validated our interpretation by studying quercetin's interaction with SDS micelles. Pyrene fluorescence and the quercetin UV-visible spectra show that the adsorption site for pyrene and quercetin in bile salt micelles is more hydrophobic than that for SDS micelles. Also, both quercetin and pyrene reported a higher critical micelle concentration for bile salts than for SDS. Our method of using a flavonoid as an intrinsic probe, is generally applicable to other lipophilic bioactives, whenever they have observable environmental dependent properties.
Keywords: Bioavailability; Micelles; Pyrene (PubChem CID: 104853); Pyrene fluorescence; Quercetin (PubChem CID: 5280804); Quercetin’s pK(a1); Sodium dodecyl sulphate (PubChem CID: 3423265); Sodium glycodeoxycholate (PubChem CID: 3035026); Sodium taurocholate (PubChem CID: 3035026); UV–visible spectra.
Copyright © 2016 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures





Similar articles
-
Towards an Understanding of the Low Bioavailability of Quercetin: A Study of Its Interaction with Intestinal Lipids.Nutrients. 2017 Feb 5;9(2):111. doi: 10.3390/nu9020111. Nutrients. 2017. PMID: 28165426 Free PMC article.
-
Beta-blockers and benzodiazepines location in SDS and bile salt micellar systems. An ESR study.J Pharm Biomed Anal. 2007 Sep 21;45(1):62-69. doi: 10.1016/j.jpba.2007.05.023. Epub 2007 May 25. J Pharm Biomed Anal. 2007. PMID: 17606356
-
Lipoamino acid-based micelles as promising delivery vehicles for monomeric amphotericin B.Int J Pharm. 2016 Jan 30;497(1-2):23-35. doi: 10.1016/j.ijpharm.2015.11.034. Epub 2015 Nov 23. Int J Pharm. 2016. PMID: 26617315
-
Separation and quantitation of cholesterol "carriers" in bile.Hepatology. 1990 Sep;12(3 Pt 2):94S-104S; discussion 104S-105S. Hepatology. 1990. PMID: 2210665 Review.
-
Drug design strategies that aim to improve the low solubility and poor bioavailability conundrum in quercetin derivatives.Expert Opin Drug Discov. 2023 Jul-Dec;18(10):1117-1132. doi: 10.1080/17460441.2023.2241366. Epub 2023 Jul 29. Expert Opin Drug Discov. 2023. PMID: 37515777 Review.
Cited by
-
In vitro antibacterial activity of photoactivated flavonoid glycosides against Acinetobacter baumannii.AMB Express. 2024 Nov 4;14(1):119. doi: 10.1186/s13568-024-01781-6. AMB Express. 2024. PMID: 39495421 Free PMC article.
-
Facile, environmentally benign and scalable approach to produce pristine few layers graphene suitable for preparing biocompatible polymer nanocomposites.Sci Rep. 2018 Jul 25;8(1):11228. doi: 10.1038/s41598-018-28560-1. Sci Rep. 2018. PMID: 30046158 Free PMC article.
-
Applying Isothermal Titration Calorimetry and Saturation Transfer Difference-NMR to Study the Mode of Interaction of Flavan-3-ols with α-Amylase to Understand Their Impact on Starch Hydrolysis.J Agric Food Chem. 2025 Apr 16;73(15):9047-9061. doi: 10.1021/acs.jafc.4c13178. Epub 2025 Apr 4. J Agric Food Chem. 2025. PMID: 40184499 Free PMC article.
-
Changes in Phenolic Profile and Total Phenol and Total Flavonoid Contents of Guadua angustifolia Kunth Plants under Organic and Conventional Fertilization.ACS Omega. 2023 Oct 25;8(44):41223-41231. doi: 10.1021/acsomega.3c04579. eCollection 2023 Nov 7. ACS Omega. 2023. PMID: 37970062 Free PMC article.
-
Absorption Coefficients of Phenolic Structures in Different Solvents Routinely Used for Experiments.Molecules. 2021 Jul 31;26(15):4656. doi: 10.3390/molecules26154656. Molecules. 2021. PMID: 34361808 Free PMC article.
References
-
- Aguiar P., Carpena J.A., Molinar-Bolivar C., Ruiz C.C. On the determination of the critical micelle concentration by the pyrene 1:3 ratio method. Journal of Colloid and Interface Science. 2003;258(1–2):116–122.
-
- Apak R., Guclu K., Ozyurek M., Karademir S.E. Novel total antioxidant index for dietary phenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. Journal of Agricultural and Food Chemistry. 2004;52(26):7979–7981. - PubMed
-
- Armstrong M.J., Carey M.C. The hydrophilic-hydrophobic balance of bile salts. Inverse correlation between reverse-phase high performance liquid chromatography and micellar cholesterol-solubilizing capacities. Journal of Lipid Research. 1982;23(1):70–80. - PubMed
-
- Azuma K., Ippoushi K., Ito H., Higashio H., Terao J. Combination of lipids and emulsifiers enhances the absorption of orally administered quercetin in rats. Journal of Agricultural and Food Chemistry. 2002;50(6):1706–1712. - PubMed
-
- Baloch M.K., Hameed G., Bano A. Effect of electrolyte concentration and temperature on the cmc of surfactants. Journal of the Chemical Society of Pakistan. 2002;24(2):77–86.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

