Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial
- PMID: 27283779
- PMCID: PMC5095784
- DOI: 10.1002/ejhf.580
Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial
Abstract
Aims: In this analysis, we utilized data from PARADIGM-HF to test the hypothesis that participants who exhibited any dose reduction during the trial would have similar benefits from lower doses of sacubitril/valsartan relative to lower doses of enalapril.
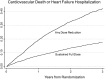
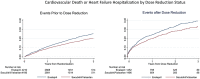
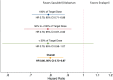
Methods and results: In a post-hoc analysis from PARADIGM-HF, we characterized patients by whether they received the maximal dose (200 mg sacubitril/valsartan or 10 mg enalapril twice daily) throughout the trial or had any dose reduction to lower doses (100/50/0 mg sacubitril/valsartan or 5/2.5/0 mg enalapril twice daily). The treatment effect for the primary outcome was estimated, stratified by dose level using time-updated Cox regression models. In the two treatment arms, participants with a dose reduction (43% of those randomized to enalapril and 42% of those randomized to sacubitril/valsartan) had similar baseline characteristics and similar baseline predictors of the need for dose reduction. In a time-updated analysis, any dose reduction was associated with a higher subsequent risk of the primary event [hazard ratio (HR) 2.5, 95% confidence interval (CI) 2.2-2.7]. However, the treatment benefit of sacubitril/valsartan over enalapril following a dose reduction was similar (HR 0.80, 95% CI 0.70-0.93, P < 0.001) to that observed in patients who had not experienced any dose reduction (HR 0.79, 95% CI 0.71-0.88, P < 0.001).
Conclusions: In PARADIGM-HF, study medication dose reduction identified patients at higher risk of a major cardiovascular event. The magnitude of benefit for patients on lower doses of sacubitril/valsartan relative to those on lower doses of enalapril was similar to that of patients who remained on target doses of both drugs.
Keywords: Chronic heart failure; Clinical trial; Neprilysin inhibitor; Sacubitril; Valsartan.
© 2016 The Authors. European Journal of Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Figures
Comment in
-
PARADIGM-HF: does dose matter?Eur J Heart Fail. 2016 Oct;18(10):1235-1237. doi: 10.1002/ejhf.634. Eur J Heart Fail. 2016. PMID: 27704710 No abstract available.
References
-
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF Investigators and Committees . Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. - PubMed
-
- Vardeny O, Miller R, Solomon SD. Combined neprilysin and renin–angiotensin system inhibition for the treatment of heart failure. JACC Heart Fail 2014;2:663–670. - PubMed
-
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM‐HF Committees and Investigators . Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin‐converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM‐HF). Eur J Heart Fail 2013;15:1062–73. - PMC - PubMed
-
- Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med 1987;316:1429–1435. - PubMed
-
- Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med 1991;325:293–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

