Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: a randomised, open-label, phase 3 trial
- PMID: 27283863
- PMCID: PMC5521044
- DOI: 10.1016/S1470-2045(16)30125-5
Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: a randomised, open-label, phase 3 trial
Erratum in
-
Correction to Lancet Oncol 2016; 17: 995, 1002.Lancet Oncol. 2016 Jul;17(7):e270. doi: 10.1016/S1470-2045(16)30232-7. Epub 2016 Jun 28. Lancet Oncol. 2016. PMID: 27733293 No abstract available.
Abstract
Background: In the phase 3 CheckMate 025 study, previously treated patients with advanced renal cell carcinoma who were randomly assigned to nivolumab had an overall survival benefit compared with those assigned to everolimus. We aimed to compare health-related quality of life (HRQoL) between treatment groups in this trial.
Methods: CheckMate 025 was an open-label study done at 146 oncology centres in 24 countries. Patients were randomly assigned to treatment between Oct 22, 2012, and March 11, 2014. Patients with advanced renal cell carcinoma were randomly assigned (1:1, block size of four) to receive nivolumab every 2 weeks or everolimus once per day. The study was stopped early at the planned interim analysis in July, 2015, because the study met its primary endpoint. A protocol amendment permitted patients in the everolimus group to cross over to nivolumab treatment. All patients not on active study therapy are being followed up for survival. At the interim analysis, HRQoL was assessed with the Functional Assessment of Cancer Therapy-Kidney Symptom Index-Disease Related Symptoms (FKSI-DRS) and European Quality of Life (EuroQol)-5 Dimensions (EQ-5D) questionnaires. Prespecified endpoints were to assess, in each treatment group, disease-related symptom progression rate based on the FKSI-DRS and changes in reported global health outcomes based on the EQ-5D. Other endpoints were post hoc. We calculated the proportion of FKSI-DRS questionnaires completed using the number of patients with non-missing data at baseline and at least one post-baseline visit. We defined FKSI-DRS completion as completion of five or more of the nine items in the questionnaire; otherwise data were treated as missing. FKSI-DRS symptom index score was prorated for missing items. We made no adjustments for missing EQ-5D data. We used descriptive statistics and multivariate analyses, including mixed-effects model repeated-measures, for between group comparisons. Analyses were powered according to the original study protocol, and we analysed FKSI-DRS and EQ-5D data for all patients who underwent randomisation and had a baseline assessment and at least one post-baseline assessment. CheckMate 025 is registered with ClinicalTrials.gov, number NCT01668784.
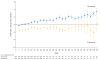
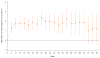
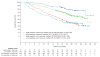
Findings: HRQoL data were collected at baseline for 362 (88%) of 410 patients in the nivolumab group and 344 (84%) of 411 patients in the everolimus group. The mean difference in FKSI-DRS scores between the nivolumab and everolimus groups was 1·6 (95% CI 1·4-1·9; p<0·0001) with descriptive statistics and 1·7 (1·2-2·1; p<0·0001) with mixed-effects model repeated-measures analysis. In terms of FKSI-DRS score, more patients had a clinically meaningful (ie, an increase of at least 2 points from baseline) HRQoL improvement with nivolumab (200 [55%] of 361 patients) versus everolimus (126 [37%] of 343 patients; p<0·0001). Median time to HRQoL improvement was shorter in patients given nivolumab (4·7 months, 95% CI 3·7-7·5) than in patients given everolimus (median not reached, NE-NE).
Interpretation: Nivolumab was associated with HRQoL improvement compared with everolimus in previously treated patients with advanced renal cell carcinoma.
Funding: Bristol-Myers Squibb.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
BB reports consulting fee from Adelphi Values for consulting provided on analyses of patient-reported outcome data, fees for participation in review activities from Adelphi Values for statistical analyses, payment for writing or reviewing from Adelphi Values for developing drafts and reviewing, and consultancy and employment at Adelphi Values outside the submitted work. EB reports employment and stock options from Bristol-Myers Squibb outside the submitted work. SB reported fees for participation in review activities from Berry Consultants as well as consultancy from Berry Consultants outside the submitted work. KB reported fees for consulting from Berry Consultants as well as consultancy from Berry Consultants outside the submitted work. DC has no interests to declare. HD reports employment and stocks with Bristol-Myers Squibb outside of the submitted work. JD reports employment and stock options from Bristol-Myers Squibb outside the submitted work. MD reports consulting fee, fees for participation in review activities, and payment for writing or reviewing, all from Adelphi Values; as well as consultancy from Adelphi Values outside the submitted work. VG reports fees for consulting as well as support for travel from Bristol-Myers Squibb, as well as consultancy from Bristol-Myers Squibb, Novartis, Pfizer, and Bayer and payment from lectures from Bristol-Myers Squibb, Novartis, and Pfizer, and travel accommodations from Bristol-Myers Squibb, Novartis, Pfizer, MSD, and Merck KGaA outside the submitted work. RJM reports a grant and travel support from Bristol-Myers Squibb, consultancy from Pfizer, Eisai, Inc and Novartis, and grants and grants pending from Pfizer, GlaxoSmithKline, Novartis, Eisai Inc, and Exelixis outside the submitted work. PN reports board membership, payment for lectures, and travel expenses from Bristol-Myers Squibb outside the submitted work. FT reports consulting, travel support, and fees for participation in review activities from Bristol-Myers Squibb to Adelphi Values, as well as consultancy and employment from Bristol-Myers Squibb to Adelphi Values outside the submitted work.
Figures

Comment in
-
Improved quality of life is the way to longer life.Lancet Oncol. 2016 Jul;17(7):862-863. doi: 10.1016/S1470-2045(16)30158-9. Epub 2016 Jun 6. Lancet Oncol. 2016. PMID: 27283861 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

