Effects of aging on mitochondrial function in skeletal muscle of American American Quarter Horses
- PMID: 27283918
- PMCID: PMC5040552
- DOI: 10.1152/japplphysiol.01077.2015
Effects of aging on mitochondrial function in skeletal muscle of American American Quarter Horses
Abstract
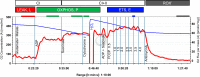
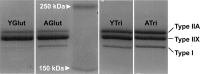
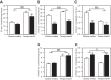
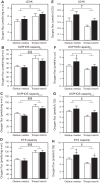
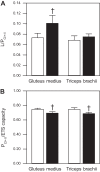
Skeletal muscle function, aerobic capacity, and mitochondrial (Mt) function have been found to decline with age in humans and rodents. However, not much is known about age-related changes in Mt function in equine skeletal muscle. Here, we compared fiber-type composition and Mt function in gluteus medius and triceps brachii muscle between young (age 1.8 ± 0.1 yr, n = 24) and aged (age 17-25 yr, n = 10) American Quarter Horses. The percentage of myosin heavy chain (MHC) IIX was lower in aged compared with young muscles (gluteus, P = 0.092; triceps, P = 0.012), while the percentages of MHC I (gluteus; P < 0.001) and MHC IIA (triceps; P = 0.023) were increased. Mass-specific Mt density, indicated by citrate synthase activity, was unaffected by age in gluteus, but decreased in aged triceps (P = 0.023). Cytochrome-c oxidase (COX) activity per milligram tissue and per Mt unit decreased with age in gluteus (P < 0.001 for both) and triceps (P < 0.001 and P = 0.003, respectively). Activity of 3-hydroxyacyl-CoA dehydrogenase per milligram tissue was unaffected by age, but increased per Mt unit in aged gluteus and triceps (P = 0.023 and P < 0.001, respectively). Mt respiration of permeabilized muscle fibers per milligram tissue was unaffected by age in both muscles. Main effects of age appeared when respiration was normalized to Mt content, with increases in LEAK, oxidative phosphorylation capacity, and electron transport system capacity (P = 0.038, P = 0.045, and P = 0.007, respectively), independent of muscle. In conclusion, equine skeletal muscle aging was accompanied by a shift in fiber-type composition, decrease in Mt density and COX activity, but preserved Mt respiratory function.
Keywords: 3-hydroxyacyl-CoA dehydrogenase; citrate synthase; cytochrome-c oxidase; high-resolution respirometry; myosin heavy chain isoform.
Copyright © 2016 the American Physiological Society.
Figures
References
-
- Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports 13: 40–47, 2003. - PubMed
-
- Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am J Physiol Cell Physiol 290: C844–C851, 2006. - PubMed
-
- Anton SD, Woods AJ, Ashizawa T, Barb D, Buford TW, Carter CS, Clark DJ, Cohen RA, Corbett DB, Cruz-Almeida Y, Dotson V, Ebner N, Efron PA, Fillingim RB, Foster TC, Gundermann DM, Joseph AM, Karabetian C, Leeuwenburgh C, Manini TM, Marsiske M, Mankowski RT, Mutchie HL, Perri MG, Ranka S, Rashidi P, Sandesara B, Scarpace PJ, Sibille KT, Solberg LM, Someya S, Uphold C, Wohlgemuth S, Wu SS, Pahor M. Successful aging: advancing the science of physical independence in older adults. Ageing Res Rev 24: 304–327, 2015. - PMC - PubMed
-
- Barrey E, Valette JP, Jouglin M, Blouin C, Langlois B. Heritability of percentage of fast myosin heavy chains in skeletal muscles and relationship with performance. Equine Vet J Suppl 30: 289–292, 1999. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

