Dual Function of Phosphoubiquitin in E3 Activation of Parkin
- PMID: 27284007
- PMCID: PMC4974400
- DOI: 10.1074/jbc.M116.728600
Dual Function of Phosphoubiquitin in E3 Activation of Parkin
Abstract
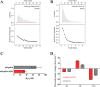
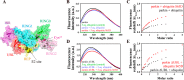
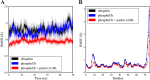
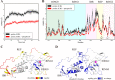
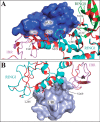
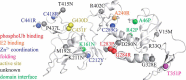
Mutations in the gene encoding parkin, an auto-inhibited E3 ubiquitin ligase that functions in the clearance of damaged mitochondria, are the most common cause of autosomal recessive juvenile Parkinsonism. The mechanism regulating parkin activation remains poorly understood. Here we show, by using isothermal titration calorimetry, solution NMR, and fluorescence spectroscopy, that parkin can bind ubiquitin and phosphomimetic ubiquitin by recognizing the canonical hydrophobic patch and C terminus of ubiquitin. The affinity of parkin for both phosphomimetic and unmodified ubiquitin is markedly enhanced upon removal of the ubiquitin-like (UBL) domain of parkin. This suggests that the agonistic binding of ubiquitin to parkin in trans is counterbalanced by the antagonistic activity of the parkin UBL domain in cis Intriguingly, UBL binding is enthalpy-driven, whereas ubiquitin binding is driven by an increase in the total entropy of the system. These thermodynamic differences are explained by different chemistry in the ubiquitin- and UBL-binding pockets of parkin and, as shown by molecular dynamics simulations, are not a consequence of changes in protein conformational entropy. Indeed, comparison of conformational fluctuations reveals that the RING1-IBR element becomes considerably more rigid upon complex formation. A model of parkin activation is proposed in which E2∼Ub binding triggers large scale diffusional motion of the RING2 domain toward the ubiquitin-stabilized RING1-IBR assembly to complete formation of the active parkin-E2∼Ub transfer complex. Thus, ubiquitin plays a dual role in parkin activation by competing with the inhibitory UBL domain and stabilizing the active form of parkin.
Keywords: Parkinson disease; isothermal titration calorimetry (ITC); molecular dynamics; parkin; protein dynamics; protein-protein interaction; ubiquitin; ubiquitin ligase.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., and Shimizu N. (1998) Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 - PubMed
-
- Shulman J. M., De Jager P. L., and Feany M. B. (2011) Parkinson's disease: genetics and pathogenesis. Annu. Rev. Pathol. 6, 193–222 - PubMed
-
- Lücking C. B., Dürr A., Bonifati V., Vaughan J., De Michele G., Gasser T., Harhangi B. S., Meco G., Denèfle P., Wood N. W., Agid Y., Brice A., French Parkinson's Disease Genetics Study Group, and European Consortium on Genetic Susceptibility in Parkinson's Disease (2000) Association between early-onset Parkinson's disease and mutations in the parkin gene. N. Engl. J. Med. 342, 1560–1567 - PubMed
-
- Matsuda N., Sato S., Shiba K., Okatsu K., Saisho K., Gautier C. A., Sou Y. S., Saiki S., Kawajiri S., Sato F., Kimura M., Komatsu M., Hattori N., and Tanaka K. (2010) PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 189, 211–221 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

