Construction of membrane-bound artificial cells using microfluidics: a new frontier in bottom-up synthetic biology
- PMID: 27284034
- PMCID: PMC4900754
- DOI: 10.1042/BST20160052
Construction of membrane-bound artificial cells using microfluidics: a new frontier in bottom-up synthetic biology
Abstract
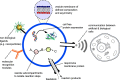
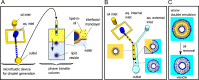
The quest to construct artificial cells from the bottom-up using simple building blocks has received much attention over recent decades and is one of the grand challenges in synthetic biology. Cell mimics that are encapsulated by lipid membranes are a particularly powerful class of artificial cells due to their biocompatibility and the ability to reconstitute biological machinery within them. One of the key obstacles in the field centres on the following: how can membrane-based artificial cells be generated in a controlled way and in high-throughput? In particular, how can they be constructed to have precisely defined parameters including size, biomolecular composition and spatial organization? Microfluidic generation strategies have proved instrumental in addressing these questions. This article will outline some of the major principles underpinning membrane-based artificial cells and their construction using microfluidics, and will detail some recent landmarks that have been achieved.
Keywords: artificial cells; biomimetics; droplet interface bilayers; microfluidics; synthetic biology; vesicles.
© 2016 The Author(s).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

