Quality of reporting of randomised controlled trials in chiropractic using the CONSORT checklist
- PMID: 27284400
- PMCID: PMC4899907
- DOI: 10.1186/s12998-016-0099-6
Quality of reporting of randomised controlled trials in chiropractic using the CONSORT checklist
Erratum in
-
Erratum to: Quality of reporting of randomised controlled trials in chiropractic using the CONSORT checklist.Chiropr Man Therap. 2016 Aug 17;24:36. doi: 10.1186/s12998-016-0120-0. eCollection 2016. Chiropr Man Therap. 2016. PMID: 27536349 Free PMC article.
Abstract
Background: Reviews indicate that the quality of reporting of randomised controlled trials (RCTs) in the medical literature is less than optimal, poor to moderate, and require improving. However, the reporting quality of chiropractic RCTs is unknown. As a result, the aim of this study was to assess the reporting quality of chiropractic RCTs and identify factors associated with better reporting quality. We hypothesized that quality of reporting of RCTs was influenced by industry funding, positive findings, larger sample sizes, latter year of publication and publication in non-chiropractic journals.
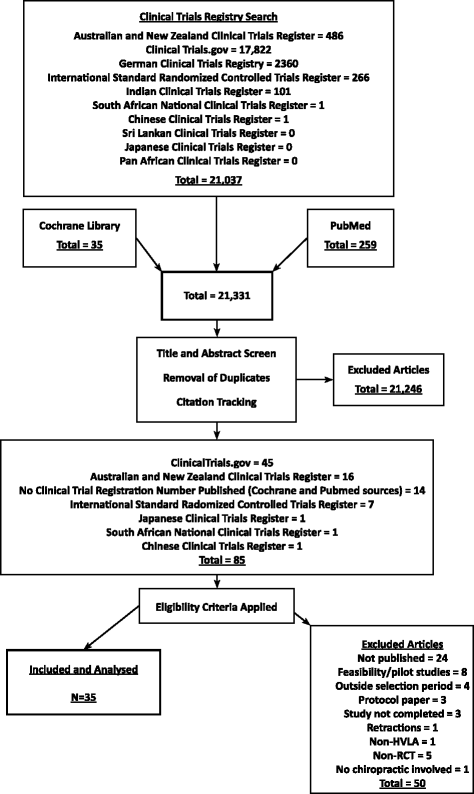
Methods: RCTs published between 2005 and 2014 were sourced from clinical trial registers, PubMed and the Cochrane Reviews. RCTs were included if they involved high-velocity, low-amplitude (HVLA) spinal and/or extremity manipulation and were conducted by a chiropractor or within a chiropractic department. Data extraction, and reviews were conducted by all authors independently. Disagreements were resolved by consensus.
Outcomes: a 39-point overall quality of reporting score checklist was developed based on the CONSORT 2010 and CONSORT for Non-Pharmacological Treatments statements. Four key methodological items, based on allocation concealment, blinding of participants and assessors, and use of intention-to-treat analysis (ITT) were also investigated.
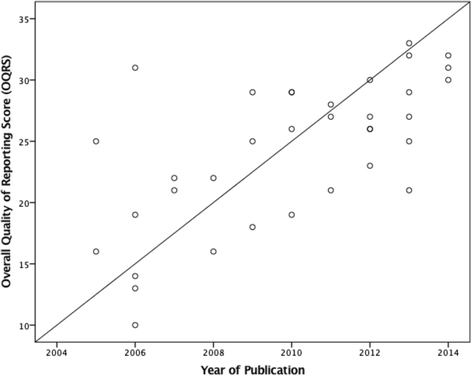
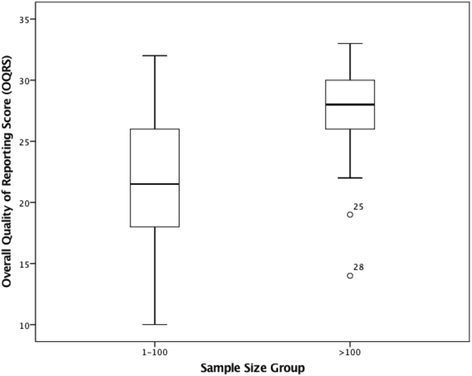
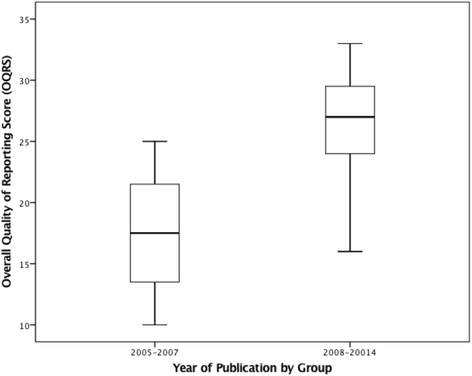
Results: Thirty-five RCTs were included. The overall quality of reporting score ranged between 10 and 33 (median score 26.0; IQR = 8.00). Allocation concealment, blinding of participants and assessors and ITT analysis were reported in 31 (87 %), 16 (46 %), 25 (71 %) and 21 (60 %) of the 35 RCTs respectively. Items most underreported were from the CONSORT for Non-Pharmacological Treatments statement. Multivariate regression analysis, revealed that year of publication (t32 = 5.17, p = 0.000, 95 % CI: 0.76, 1.76), and sample size (t32 = 3.01, p = 0.005, 95 % CI: 1.36, 7.02), were the only two factors associated with reporting quality.
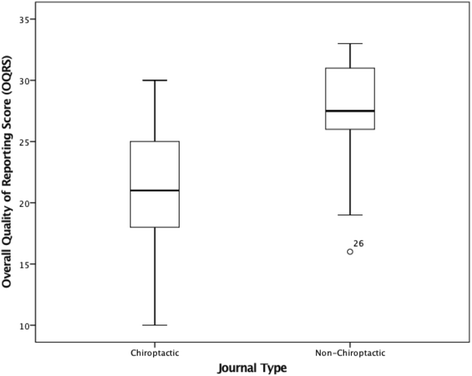
Conclusion: The overall quality of reporting RCTs in chiropractic ranged from poor to excellent, improving between 2005 and 2014. This study suggests that quality of reporting, was influenced by year of publication and sample size but not journal type, funding source or outcome positivity. Reporting of some key methodological items and uptake of items from the CONSORT Extension for Non-Pharmacological Treatments items was suboptimal. Future recommendations were made.
Keywords: Chiropractic manipulation; Manipulation; Musculoskeletal; Quality of reporting; Randomised controlled trials; Spinal manipulative therapy; Spine; The CONSORT statement.
Figures
Similar articles
-
Quality of reporting for randomized controlled trials in the hypospadias literature: Where do we stand?J Pediatr Urol. 2017 Oct;13(5):482.e1-482.e9. doi: 10.1016/j.jpurol.2017.03.031. Epub 2017 Apr 24. J Pediatr Urol. 2017. PMID: 28566206
-
Reporting Quality of Randomized Controlled Trials of Periodontal Diseases in Journal Abstracts-A Cross-sectional Survey and Bibliometric Analysis.J Evid Based Dent Pract. 2018 Jun;18(2):130-141.e22. doi: 10.1016/j.jebdp.2017.08.005. Epub 2017 Sep 21. J Evid Based Dent Pract. 2018. PMID: 29747793
-
Quality of reporting for pilot randomized controlled trials in the pediatric urology literature-A systematic review.J Pediatr Urol. 2021 Dec;17(6):846-854. doi: 10.1016/j.jpurol.2021.09.012. Epub 2021 Sep 24. J Pediatr Urol. 2021. PMID: 34635440
-
The quality of reporting of RCTs used within a postoperative pain management meta-analysis, using the CONSORT statement.BMC Anesthesiol. 2012 Jul 4;12:13. doi: 10.1186/1471-2253-12-13. BMC Anesthesiol. 2012. PMID: 22762351 Free PMC article.
-
The Deficits of the Methodological and Reporting Quality of Randomized Controlled Trials in the Field of Prosthetics and Orthotics in Iran: A Systematic Review.Rev Recent Clin Trials. 2023;18(2):92-111. doi: 10.2174/1574887118666230221114201. Rev Recent Clin Trials. 2023. PMID: 36809948
Cited by
-
Compliance of Published Randomized Controlled Trials on the Effect of Physical Activity on Primary Dysmenorrhea with the Consortium's Integrated Report on Clinical Trials Statement: A Critical Appraisal of the Literature.Iran J Nurs Midwifery Res. 2020 Nov 7;25(6):445-454. doi: 10.4103/ijnmr.IJNMR_223_19. eCollection 2020 Nov-Dec. Iran J Nurs Midwifery Res. 2020. PMID: 33747832 Free PMC article. Review.
-
Care Outcomes for Chiropractic Outpatient Veterans (COCOV): a qualitative study with veteran stakeholders from a pilot trial of multimodal chiropractic care.Pilot Feasibility Stud. 2022 Jan 14;8(1):6. doi: 10.1186/s40814-021-00962-5. Pilot Feasibility Stud. 2022. PMID: 35031072 Free PMC article.
-
Erratum to: Quality of reporting of randomised controlled trials in chiropractic using the CONSORT checklist.Chiropr Man Therap. 2016 Aug 17;24:36. doi: 10.1186/s12998-016-0120-0. eCollection 2016. Chiropr Man Therap. 2016. PMID: 27536349 Free PMC article.
-
The critical appraisal of randomized controlled trials published in an Indian journal to assess the quality of reporting: A retrospective, cross-sectional study.Perspect Clin Res. 2022 Jan-Mar;13(1):33-37. doi: 10.4103/picr.PICR_169_19. Epub 2021 Jan 8. Perspect Clin Res. 2022. PMID: 35198426 Free PMC article.
-
Assessment of reporting quality of randomized controlled trials investigating the effects of inulin-type fructans supplementation on cardiovascular disease risk factors: A systematic survey.PLoS One. 2024 Jan 2;19(1):e0292184. doi: 10.1371/journal.pone.0292184. eCollection 2024. PLoS One. 2024. PMID: 38166017 Free PMC article.
References
-
- Cartwright N. Are RCTs the gold standard? BioSocieties. 2007;2:11–20. doi: 10.1017/S1745855207005029. - DOI
-
- Keech A, Gebski V, Pike R. Interpreting and reporting clinical trials. A guide to the CONSORT statement and the principles of randomised controlled trials. Sydney: MJA Books, Australasian Medical Publishing Company; 2007.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

