Is early response by (18)F-2-fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography a predictor of long-term outcome in patients with metastatic colorectal cancer?
- PMID: 27284468
- PMCID: PMC4880772
- DOI: 10.21037/jgo.2016.02.04
Is early response by (18)F-2-fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography a predictor of long-term outcome in patients with metastatic colorectal cancer?
Abstract
Background: Identify in advance responder patients to chemotherapy in metastatic colorectal cancer (CRC) would allow prompt interruption of ineffective therapies in non-responder patients. Hence, predictive markers are sought in numerous trials to detect responder patients, including tumor shrinkage measured by imaging methods. Usually, Response Evaluation Criteria in Solid Tumors (RECIST) is used to evaluate tumor response in metastatic CRC, but these criteria are questionable with use of biological agents associated to chemotherapy. Our aim was correlate early metabolic response by (18)F-2-fluoro-2-deoxy-D-glucose positron emission tomography-computed tomography ((18)FDG-PET-CT) with long-term outcome in metastatic CRC in first-line therapy.
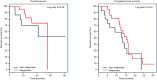
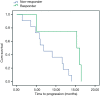
Methods: We prospectively evaluated 36 patients with metastatic CRC in first-line treatment with 5-fluorouracil, leucovorin (folinic acid), oxaliplatin (FOLFOX) or 5-fluorouracil, leucovorin (folinic acid), irinotecan (FOLFIRI) associated with cetuximab or bevacizumab. (18)FDG-PET-CT was performed at baseline and after two cycles of chemotherapy. The early metabolic response [standardized uptake value (SUV)] was measured to identify responder and non-responder patients and correlated with overall survival (OS) and progression-free survival (PFS).
Results: Median age was 58.5 years (range, 41-74 years). PFS was 15.5 months for responder and 13.3 months for non-responder (P=0.42), OS was 55.7 months for responder and not reached for non-responder. There was no correlation between delta-SUV and clinical and pathological variables analyzed. In the subgroup of patients who did not undergo resection of metastasis (45%), PFS was higher for responders (15.3×6.8 months, P=0.02).
Conclusions: According to our findings, early response by (18)FDG-PET-CT was not a predictor of long-term outcome for patients with metastatic CRC treated in the first-line chemotherapy with a monoclonal antibody.
Keywords: Metabolic response; colorectal cancer (CRC); positron emission tomography.
Conflict of interest statement
Figures
Similar articles
-
3'-Deoxy-3'-18F-Fluorothymidine and 18F-Fluorodeoxyglucose positron emission tomography for the early prediction of response to Regorafenib in patients with metastatic colorectal cancer refractory to all standard therapies.Eur J Nucl Med Mol Imaging. 2019 Jul;46(8):1713-1722. doi: 10.1007/s00259-019-04330-7. Epub 2019 Apr 30. Eur J Nucl Med Mol Imaging. 2019. PMID: 31041456
-
Early PET/CT scan is more effective than RECIST in predicting outcome of patients with liver metastases from colorectal cancer treated with preoperative chemotherapy plus bevacizumab.J Nucl Med. 2013 Dec;54(12):2062-9. doi: 10.2967/jnumed.113.119909. Epub 2013 Oct 17. J Nucl Med. 2013. PMID: 24136935
-
Impact of Fluorine-18 2-Fluoro-2-Deoxy-D-Glucose Uptake on Preoperative Positron Emission Tomography/Computed Tomography in the Lymph Nodes of Patients with Primary Colorectal Cancer.Dig Surg. 2017;34(1):60-67. doi: 10.1159/000448222. Epub 2016 Jul 26. Dig Surg. 2017. PMID: 27454870
-
Chemotherapy of metastatic colorectal cancer.Prescrire Int. 2010 Oct;19(109):219-24. Prescrire Int. 2010. PMID: 21180382 Review.
-
[Recent results of irinotecan therapy in colorectal cancer].Magy Onkol. 2004;48(4):281-8. Epub 2005 Jan 17. Magy Onkol. 2004. PMID: 15655572 Review. Hungarian.
Cited by
-
Anti-allodynic effect of Buja in a rat model of oxaliplatin-induced peripheral neuropathy via spinal astrocytes and pro-inflammatory cytokines suppression.BMC Complement Altern Med. 2017 Jan 14;17(1):48. doi: 10.1186/s12906-017-1556-z. BMC Complement Altern Med. 2017. PMID: 28088201 Free PMC article.
References
-
- American Cancer Society: Cancer Facts and Figures 2015. Atlanta, Ga: American Cancer Society, 2015. Available online: http://www.cancer.org/acs/groups/content/@editorial/documents/document/a..., acessed on October 30, 2015.
-
- de Gramont A, Bosset JF, Milan C, et al. Randomized trial comparing monthly low-dose leucovorin and fluorouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol 1997;15:808-15. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
