Neonatal Masculinization Blocks Increased Excitatory Synaptic Input in Female Rat Nucleus Accumbens Core
- PMID: 27285859
- PMCID: PMC4967116
- DOI: 10.1210/en.2016-1160
Neonatal Masculinization Blocks Increased Excitatory Synaptic Input in Female Rat Nucleus Accumbens Core
Abstract
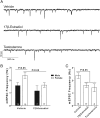
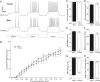
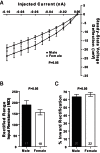
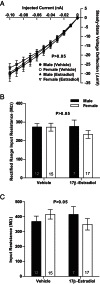
Steroid sex hormones and genetic sex regulate the phenotypes of motivated behaviors and relevant disorders. Most studies seeking to elucidate the underlying neuroendocrine mechanisms have focused on how 17β-estradiol modulates the role of dopamine in striatal brain regions, which express membrane-associated estrogen receptors. Dopamine action is an important component of striatal function, but excitatory synaptic neurotransmission has also emerged as a key striatal substrate and target of estradiol action. Here, we focus on excitatory synaptic input onto medium spiny neurons (MSNs) in the striatal region nucleus accumbens core (AcbC). In adult AcbC, miniature excitatory postsynaptic current (mEPSC) frequency is increased in female compared with male MSNs. We tested whether increased mEPSC frequency in female MSNs exists before puberty, whether this increased excitability is due to the absence of estradiol or testosterone during the early developmental critical period, and whether it is accompanied by stable neuron intrinsic membrane properties. We found that mEPSC frequency is increased in female compared with male MSNs before puberty. Increased mEPSC frequency in female MSNs is abolished after neonatal estradiol or testosterone exposure. MSN intrinsic membrane properties did not differ by sex. These data indicate that neonatal masculinization via estradiol and/or testosterone action is sufficient for down-regulating excitatory synaptic input onto MSNs. We conclude that excitatory synaptic input onto AcbC MSNs is organized long before adulthood via steroid sex hormone action, providing new insight into a mechanism by which sex differences in motivated behavior and other AbcC functions may be generated or compromised.
Figures



Similar articles
-
Estradiol rapidly modulates excitatory synapse properties in a sex- and region-specific manner in rat nucleus accumbens core and caudate-putamen.J Neurophysiol. 2019 Sep 1;122(3):1213-1225. doi: 10.1152/jn.00264.2019. Epub 2019 Jul 17. J Neurophysiol. 2019. PMID: 31314648 Free PMC article.
-
Sex Differences in Medium Spiny Neuron Excitability and Glutamatergic Synaptic Input: Heterogeneity Across Striatal Regions and Evidence for Estradiol-Dependent Sexual Differentiation.Front Endocrinol (Lausanne). 2018 Apr 18;9:173. doi: 10.3389/fendo.2018.00173. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29720962 Free PMC article. Review.
-
Differential and synergistic roles of 17β-estradiol and progesterone in modulating adult female rat nucleus accumbens core medium spiny neuron electrophysiology.J Neurophysiol. 2020 Jun 1;123(6):2390-2405. doi: 10.1152/jn.00157.2020. Epub 2020 May 13. J Neurophysiol. 2020. PMID: 32401164 Free PMC article.
-
Estrous cycle-induced sex differences in medium spiny neuron excitatory synaptic transmission and intrinsic excitability in adult rat nucleus accumbens core.J Neurophysiol. 2018 Sep 1;120(3):1356-1373. doi: 10.1152/jn.00263.2018. Epub 2018 Jun 27. J Neurophysiol. 2018. PMID: 29947588 Free PMC article.
-
Sex steroid hormones, the estrous cycle, and rapid modulation of glutamatergic synapse properties in the striatal brain regions with a focus on 17β-estradiol and the nucleus accumbens.Steroids. 2024 Jan;201:109344. doi: 10.1016/j.steroids.2023.109344. Epub 2023 Nov 17. Steroids. 2024. PMID: 37979822 Free PMC article. Review.
Cited by
-
Effects of prenatal opioid exposure on synaptic adaptations and behaviors across development.Neuropharmacology. 2023 Jan 1;222:109312. doi: 10.1016/j.neuropharm.2022.109312. Epub 2022 Nov 2. Neuropharmacology. 2023. PMID: 36334764 Free PMC article. Review.
-
Persistent Neuroadaptations in the Nucleus Accumbens Core Accompany Incubation of Methamphetamine Craving in Male and Female Rats.eNeuro. 2023 Mar 14;10(3):ENEURO.0480-22.2023. doi: 10.1523/ENEURO.0480-22.2023. Print 2023 Mar. eNeuro. 2023. PMID: 36792361 Free PMC article.
-
ERα Stimulation Rapidly Modulates Excitatory Synapse Properties in Female Rat Nucleus Accumbens Core.Neuroendocrinology. 2023;113(11):1140-1153. doi: 10.1159/000529571. Epub 2023 Feb 6. Neuroendocrinology. 2023. PMID: 36746131 Free PMC article.
-
Temporal and bidirectional influences of estradiol on voluntary wheel running in adult female and male rats.Horm Behav. 2020 Apr;120:104694. doi: 10.1016/j.yhbeh.2020.104694. Epub 2020 Jan 27. Horm Behav. 2020. PMID: 31978389 Free PMC article.
-
Biological Sex, Estradiol and Striatal Medium Spiny Neuron Physiology: A Mini-Review.Front Cell Neurosci. 2018 Dec 12;12:492. doi: 10.3389/fncel.2018.00492. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30618639 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

