Asymmetric cell division in plants: mechanisms of symmetry breaking and cell fate determination
- PMID: 27286799
- PMCID: PMC5522748
- DOI: 10.1007/s00018-016-2290-2
Asymmetric cell division in plants: mechanisms of symmetry breaking and cell fate determination
Abstract
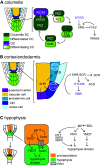
Asymmetric cell division is a fundamental mechanism that generates cell diversity while maintaining self-renewing stem cell populations in multicellular organisms. Both intrinsic and extrinsic mechanisms underpin symmetry breaking and differential daughter cell fate determination in animals and plants. The emerging picture suggests that plants deal with the problem of symmetry breaking using unique cell polarity proteins, mobile transcription factors, and cell wall components to influence asymmetric divisions and cell fate. There is a clear role for altered auxin distribution and signaling in distinguishing two daughter cells and an emerging role for epigenetic modifications through chromatin remodelers and DNA methylation in plant cell differentiation. The importance of asymmetric cell division in determining final plant form provides the impetus for its study in the areas of both basic and applied science.
Keywords: Asymmetric cell division; Cell fate determination; Cell polarity; Plant development; Stem cells.
Figures



Similar articles
-
Polarity in plant asymmetric cell division: Division orientation and cell fate differentiation.Dev Biol. 2016 Nov 1;419(1):121-131. doi: 10.1016/j.ydbio.2016.07.020. Epub 2016 Jul 28. Dev Biol. 2016. PMID: 27475487 Free PMC article. Review.
-
Symmetry breaking in plants: molecular mechanisms regulating asymmetric cell divisions in Arabidopsis.Cold Spring Harb Perspect Biol. 2009 Nov;1(5):a000497. doi: 10.1101/cshperspect.a000497. Cold Spring Harb Perspect Biol. 2009. PMID: 20066115 Free PMC article. Review.
-
The BASL polarity protein controls a MAPK signaling feedback loop in asymmetric cell division.Dev Cell. 2015 Apr 20;33(2):136-49. doi: 10.1016/j.devcel.2015.02.022. Epub 2015 Apr 2. Dev Cell. 2015. PMID: 25843888 Free PMC article.
-
Unraveling the role of epigenetic regulation in asymmetric cell division during plant development.J Exp Bot. 2022 Jan 5;73(1):38-49. doi: 10.1093/jxb/erab421. J Exp Bot. 2022. PMID: 34518884
-
The chemical compound bubblin induces stomatal mispatterning in Arabidopsis by disrupting the intrinsic polarity of stomatal lineage cells.Development. 2017 Feb 1;144(3):499-506. doi: 10.1242/dev.145458. Epub 2017 Jan 13. Development. 2017. PMID: 28087627
Cited by
-
From birth to function: Male gametophyte development in flowering plants.Curr Opin Plant Biol. 2021 Oct;63:102118. doi: 10.1016/j.pbi.2021.102118. Epub 2021 Oct 5. Curr Opin Plant Biol. 2021. PMID: 34625367 Free PMC article. Review.
-
Auxin signaling: a big question to be addressed by small molecules.J Exp Bot. 2018 Jan 4;69(2):313-328. doi: 10.1093/jxb/erx375. J Exp Bot. 2018. PMID: 29237069 Free PMC article. Review.
-
Migration of prospindle before the first asymmetric division in germinating spore of Marchantia polymorpha.Plant Biotechnol (Tokyo). 2022 Mar 25;39(1):5-12. doi: 10.5511/plantbiotechnology.21.1217b. Plant Biotechnol (Tokyo). 2022. PMID: 35800969 Free PMC article.
-
The Rab Geranylgeranyl Transferase Beta Subunit Is Essential for Embryo and Seed Development in Arabidopsis thaliana.Int J Mol Sci. 2021 Jul 24;22(15):7907. doi: 10.3390/ijms22157907. Int J Mol Sci. 2021. PMID: 34360673 Free PMC article.
-
Geometric cues forecast the switch from two- to three-dimensional growth in Physcomitrella patens.New Phytol. 2020 Mar;225(5):1945-1955. doi: 10.1111/nph.16276. Epub 2019 Dec 3. New Phytol. 2020. PMID: 31639220 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

