Synaptophysin depletion and intraneuronal Aβ in organotypic hippocampal slice cultures from huAPP transgenic mice
- PMID: 27287430
- PMCID: PMC4903008
- DOI: 10.1186/s13024-016-0110-7
Synaptophysin depletion and intraneuronal Aβ in organotypic hippocampal slice cultures from huAPP transgenic mice
Abstract
Background: To date, there are no effective disease-modifying treatments for Alzheimer's disease (AD). In order to develop new therapeutics for stages where they are most likely to be effective, it is important to identify the first pathological alterations in the disease cascade. Changes in Aβ concentration have long been reported as one of the first steps, but understanding the source, and earliest consequences, of pathology requires a model system that represents all major CNS cell types, is amenable to repeated observation and sampling, and can be readily manipulated. In this regard, long term organotypic hippocampal slice cultures (OHSCs) from neonatal amyloid mice offer an excellent compromise between in vivo and primary culture studies, largely retaining the cellular composition and neuronal architecture of the in vivo hippocampus, but with the in vitro advantages of accessibility to live imaging, sampling and intervention.
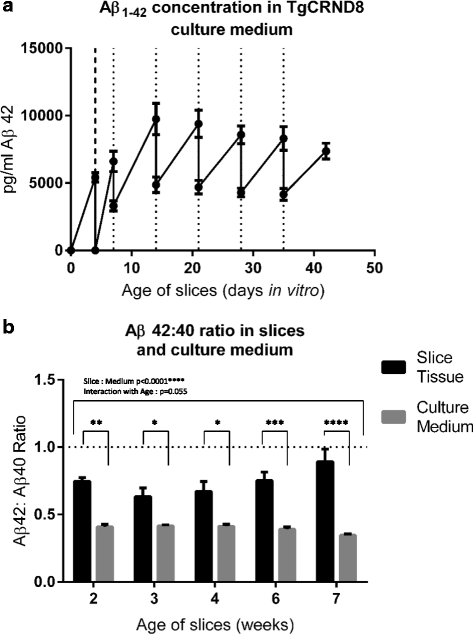
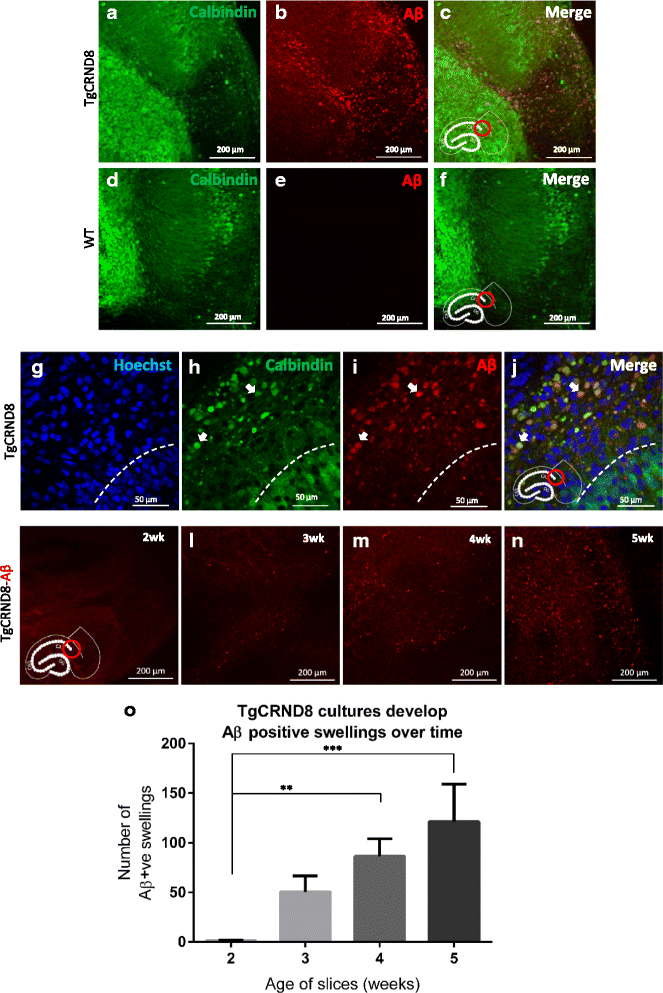
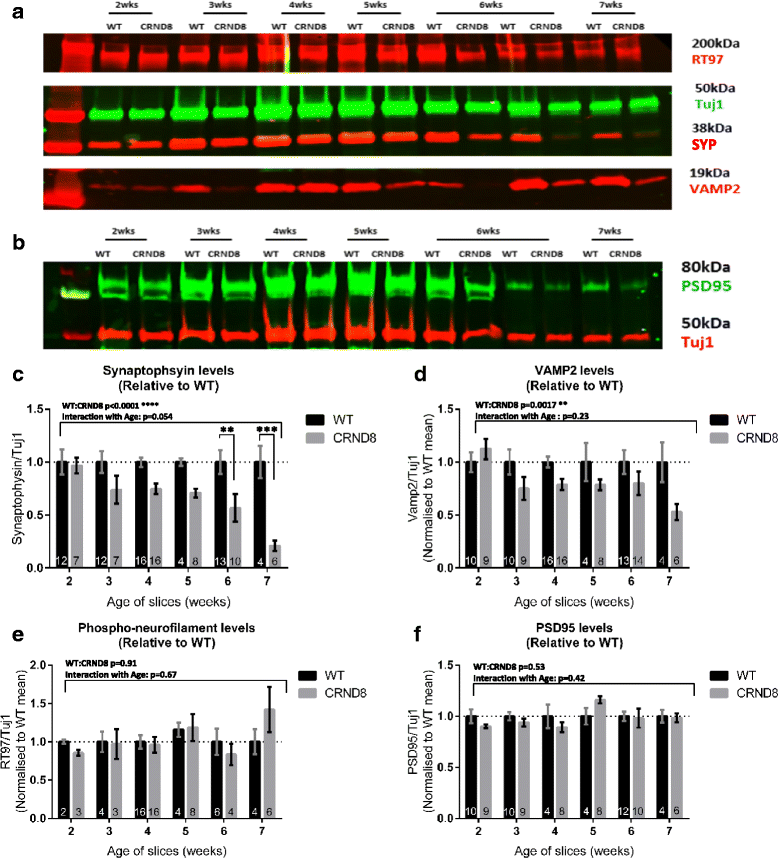
Results: Here, we report the development and characterisation of progressive pathological changes in an organotypic model from TgCRND8 mice. Aβ1-40 and Aβ1-42 rise progressively in transgenic slice culture medium and stabilise when regular feeding balances continued production. In contrast, intraneuronal Aβ continues to accumulate in close correlation with a specific decline in presynaptic proteins and puncta. Plaque pathology is not evident even when Aβ1-42 is increased by pharmacological manipulation (using calpain inhibitor 1), indicating that soluble Aβ species, or other APP processing products, are sufficient to cause the initial synaptic changes.
Conclusions: Organotypic brain slices from TgCRND8 mice represent an important new system for understanding mechanisms of Aβ generation, release and progressive toxicity. The pathology observed in these cultures will allow for rapid assessment of disease modifying compounds in a system amenable to manipulation and observation.
Keywords: Alzheimer’s disease; Amyloid; Intraneuronal Aβ; Organotypic brain slice; Synapses; TgCRND8.
Figures



Similar articles
-
Forebrain microglia from wild-type but not adult 5xFAD mice prevent amyloid-β plaque formation in organotypic hippocampal slice cultures.Sci Rep. 2015 Sep 29;5:14624. doi: 10.1038/srep14624. Sci Rep. 2015. PMID: 26416689 Free PMC article.
-
Knockout of Toll-like receptor 2 attenuates Aβ25-35-induced neurotoxicity in organotypic hippocampal slice cultures.Neurochem Int. 2013 Dec;63(8):818-25. doi: 10.1016/j.neuint.2013.10.007. Epub 2013 Oct 23. Neurochem Int. 2013. PMID: 24161618
-
Abeta-degrading endopeptidase, neprilysin, in mouse brain: synaptic and axonal localization inversely correlating with Abeta pathology.Neurosci Res. 2002 May;43(1):39-56. doi: 10.1016/s0168-0102(02)00015-9. Neurosci Res. 2002. PMID: 12074840
-
Targeting small Abeta oligomers: the solution to an Alzheimer's disease conundrum?Trends Neurosci. 2001 Apr;24(4):219-24. doi: 10.1016/s0166-2236(00)01749-5. Trends Neurosci. 2001. PMID: 11250006 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Closing the Critical Period Is Required for the Maturation of Binocular Integration in Mouse Primary Visual Cortex.Front Cell Neurosci. 2021 Nov 26;15:749265. doi: 10.3389/fncel.2021.749265. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34899187 Free PMC article.
-
Spreading of P301S Aggregated Tau Investigated in Organotypic Mouse Brain Slice Cultures.Biomolecules. 2022 Aug 23;12(9):1164. doi: 10.3390/biom12091164. Biomolecules. 2022. PMID: 36139003 Free PMC article.
-
Transgenic Mouse Models of Alzheimer's Disease: An Integrative Analysis.Int J Mol Sci. 2022 May 12;23(10):5404. doi: 10.3390/ijms23105404. Int J Mol Sci. 2022. PMID: 35628216 Free PMC article. Review.
-
Modeling Herpes Simplex Virus 1 Infections in Human Central Nervous System Neuronal Cells Using Two- and Three-Dimensional Cultures Derived from Induced Pluripotent Stem Cells.J Virol. 2019 Apr 17;93(9):e00111-19. doi: 10.1128/JVI.00111-19. Print 2019 May 1. J Virol. 2019. PMID: 30787148 Free PMC article.
-
Inhibition of PKCδ reduces amyloid-β levels and reverses Alzheimer disease phenotypes.J Exp Med. 2018 Jun 4;215(6):1665-1677. doi: 10.1084/jem.20171193. Epub 2018 May 8. J Exp Med. 2018. PMID: 29739836 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

