Safety of a single low-dose of primaquine in addition to standard artemether-lumefantrine regimen for treatment of acute uncomplicated Plasmodium falciparum malaria in Tanzania
- PMID: 27287612
- PMCID: PMC4901409
- DOI: 10.1186/s12936-016-1341-3
Safety of a single low-dose of primaquine in addition to standard artemether-lumefantrine regimen for treatment of acute uncomplicated Plasmodium falciparum malaria in Tanzania
Abstract
Background: This study assessed the safety of the new World Health Organization (WHO) recommendation of adding a single low-dose of primaquine (PQ) to standard artemisinin-based combination therapy (ACT), regardless of individual glucose-6-phosphate dehydrogenase (G6PD) status, for treatment of acute uncomplicated Plasmodium falciparum malaria in Tanzania.
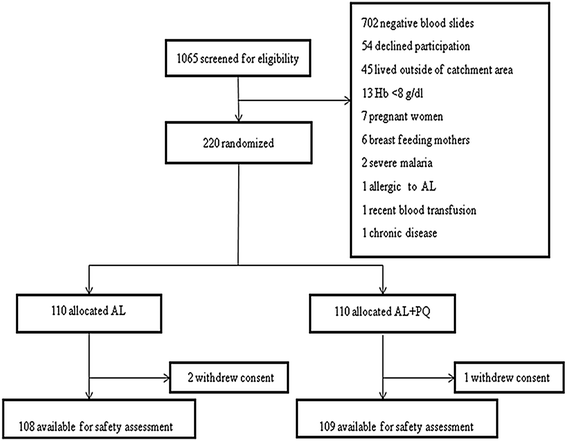
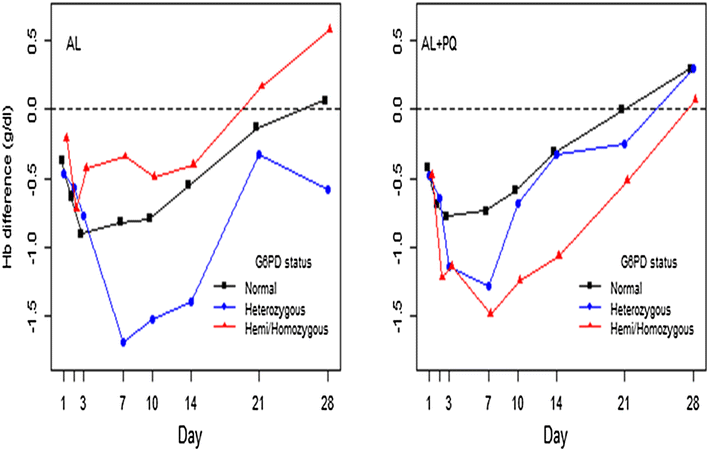
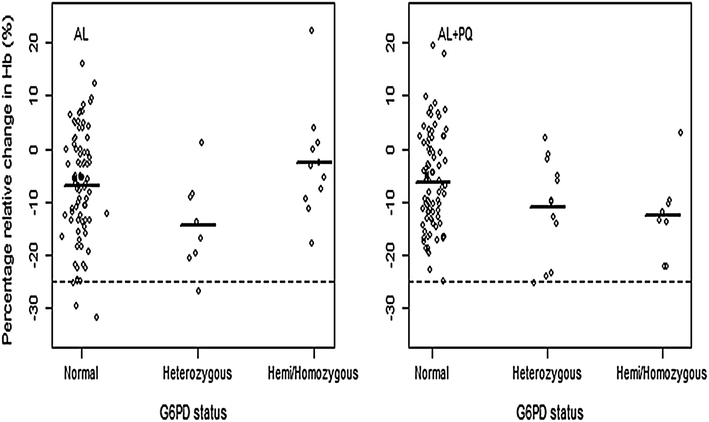
Methods: Men and non-pregnant, non-lactating women aged ≥1 year with uncomplicated P. falciparum malaria were enrolled and randomized to either standard artemether-lumefantrine (AL) regimen alone or with a 0.25 mg/kg single-dose of PQ. PQ was administered concomitantly with the first AL dose. All drug doses were supervised. Safety was evaluated between days 0 and 28. G6PD status was assessed using rapid test (CareStart™) and molecular genotyping. The primary endpoint was mean percentage relative reduction in haemoglobin (Hb) concentration (g/dL) between days 0 and 7 by genotypic G6PD status and treatment arm.
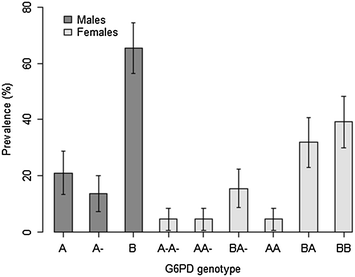
Results: Overall, 220 patients, 110 per treatment arm, were enrolled, of whom 33/217 (15.2 %) were phenotypically G6PD deficient, whereas 15/110 (13.6 %) were genotypically hemizygous males, 5/110 (4.5 %) homozygous females and 22/110 (20 %) heterozygous females. Compared to genotypically G6PD wild-type/normal [6.8, 95 % confidence interval (CI) 4.67-8.96], only heterozygous patients in AL arm had significant reduction in day-7 mean relative Hb concentration (14.3, 95 % CI 7.02-21.55, p=0.045), however, none fulfilled the pre-defined haemolytic threshold value of ≥25 % Hb reduction. After adjustment for baseline parasitaemia, Hb, age and sex the mean relative Hb reduction was not statistically significant in both heterozygous and hemizygous/homozygous patients in both arms. A majority of the adverse events (AEs) were mild and unrelated to the study drugs. However, six (4.4 %) episodes, three per treatment arm, of acute haemolytic anaemia occurred between days 0 and 7. Three occurred in phenotypically G6PD deficient patients, two in AL and one in AL + PQ arm, but none in genotypically hemizygous/homozygous patients. All patients with acute haemolytic anaemia recovered without medical intervention.
Conclusion: The findings support that the WHO recommendation of adding a single low-dose of PQ to standard AL regimen is safe for the treatment of acute uncomplicated P. falciparum malaria regardless of G6PD status in Tanzania. Trial registration number NCT02090036.
Keywords: Anaemia; Glucose-6-phosphate dehydrogenase; Plasmodium falciparum malaria; Primaquine.
Figures
Similar articles
-
Adding a single low-dose of primaquine (0.25 mg/kg) to artemether-lumefantrine did not compromise treatment outcome of uncomplicated Plasmodium falciparum malaria in Tanzania: a randomized, single-blinded clinical trial.Malar J. 2016 Aug 26;15(1):435. doi: 10.1186/s12936-016-1430-3. Malar J. 2016. PMID: 27565897 Free PMC article. Clinical Trial.
-
Development of a pharmacovigilance safety monitoring tool for the rollout of single low-dose primaquine and artemether-lumefantrine to treat Plasmodium falciparum infections in Swaziland: a pilot study.Malar J. 2016 Jul 22;15(1):384. doi: 10.1186/s12936-016-1410-7. Malar J. 2016. PMID: 27450652 Free PMC article.
-
The effect of single low-dose primaquine treatment for uncomplicated Plasmodium falciparum malaria on haemoglobin levels in Ethiopia: a longitudinal cohort study.Malar J. 2024 Jul 12;23(1):208. doi: 10.1186/s12936-024-05021-x. Malar J. 2024. PMID: 38997771 Free PMC article.
-
A systematic review of the safety and efficacy of artemether-lumefantrine against uncomplicated Plasmodium falciparum malaria during pregnancy.Malar J. 2012 May 1;11:141. doi: 10.1186/1475-2875-11-141. Malar J. 2012. PMID: 22548983 Free PMC article.
-
Safety of single-dose primaquine as a Plasmodium falciparum gametocytocide: a systematic review and meta-analysis of individual patient data.BMC Med. 2022 Sep 16;20(1):350. doi: 10.1186/s12916-022-02504-z. BMC Med. 2022. PMID: 36109733 Free PMC article.
Cited by
-
Safety of primaquine given to people with G6PD deficiency: systematic review of prospective studies.Malar J. 2017 Aug 22;16(1):346. doi: 10.1186/s12936-017-1989-3. Malar J. 2017. PMID: 28830424 Free PMC article.
-
Prevalence of and Risk Factors Associated with Polymerase Chain Reaction-Determined Plasmodium falciparum Positivity on Day 3 after Initiation of Artemether-Lumefantrine Treatment for Uncomplicated Malaria in Bagamoyo District, Tanzania.Am J Trop Med Hyg. 2019 May;100(5):1179-1186. doi: 10.4269/ajtmh.18-0729. Am J Trop Med Hyg. 2019. PMID: 30860013 Free PMC article.
-
High efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated falciparum malaria in Muheza and Kigoma Districts, Tanzania.Malar J. 2018 Jul 11;17(1):261. doi: 10.1186/s12936-018-2409-z. Malar J. 2018. PMID: 29996849 Free PMC article. Clinical Trial.
-
The tolerability of single low dose primaquine in glucose-6-phosphate deficient and normal falciparum-infected Cambodians.BMC Infect Dis. 2019 Mar 12;19(1):250. doi: 10.1186/s12879-019-3862-1. BMC Infect Dis. 2019. PMID: 30871496 Free PMC article. Clinical Trial.
-
Safety monitoring experience of single-low dose primaquine co-administered with artemether-lumefantrine among providers and patients in routine healthcare practice: a qualitative study in Eastern Tanzania.Malar J. 2021 Oct 9;20(1):392. doi: 10.1186/s12936-021-03921-w. Malar J. 2021. PMID: 34627236 Free PMC article.
References
-
- WHO . World malaria report 2014. Geneva: World Health Organization; 2014.
-
- Recht J, Ashley E, White N. Safety of 8-aminoquinoline antimalarial medicines. Geneva: World Health Organization; 2014.
-
- Graves PM, Gelband H, Garner P. Primaquine for reducing Plasmodium falciparum transmission. Cochrane database Syst Rev. 2012;9:CD008152. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

