ApoE deficiency exacerbates the development and sustainment of a semi-chronic K/BxN serum transfer-induced arthritis model
- PMID: 27287704
- PMCID: PMC4901400
- DOI: 10.1186/s12967-016-0912-y
ApoE deficiency exacerbates the development and sustainment of a semi-chronic K/BxN serum transfer-induced arthritis model
Abstract
Background: The risk for developing cardiovascular disease is greater in patients with rheumatoid arthritis (RA) than in the general population. While patients with RA also have dyslipidemia, the impact of dyslipidemia on the severity of inflammatory arthritis and associated cardiovascular disease is unclear. Currently, there are conflicting results regarding arthritis incidence in apolipoprotein E (ApoE) deficient mice, which spontaneously exhibit both hyperlipidemia and atherosclerosis. Here, we utilize a distinct approach to investigate the contribution of a hyperlipidemic environment on the development of arthritis and atherosclerosis in mice lacking ApoE.
Methods: K/BxN serum transfer-induced arthritis (STIA) was assessed in C57BL/6 (control) and ApoE(-/-) mice using clinical indices and immunohistochemical staining. Ankle synoviums were processed for flow cytometry. Aortic atherosclerosis was quantitated using Sudan IV staining. Serum cholesterol and cytokine levels were determined via enzymatic and luminex bead-based assays, respectively.
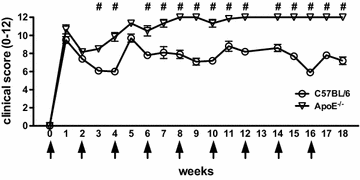
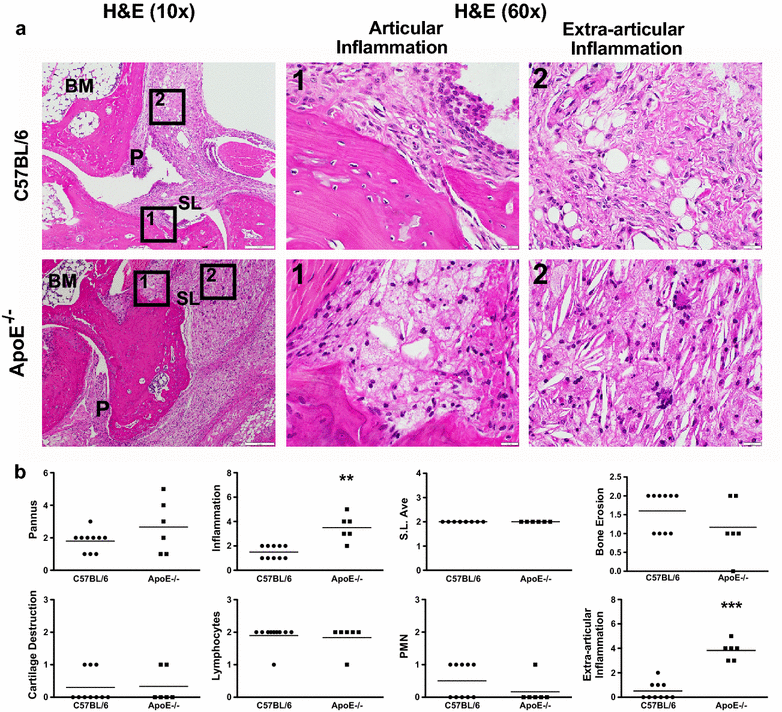
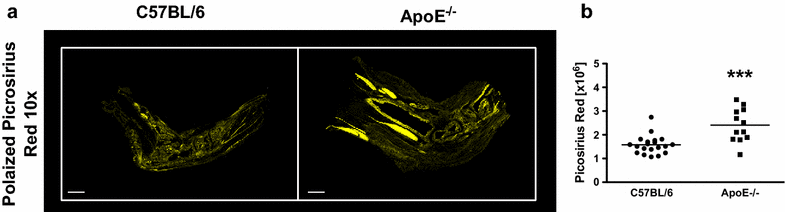
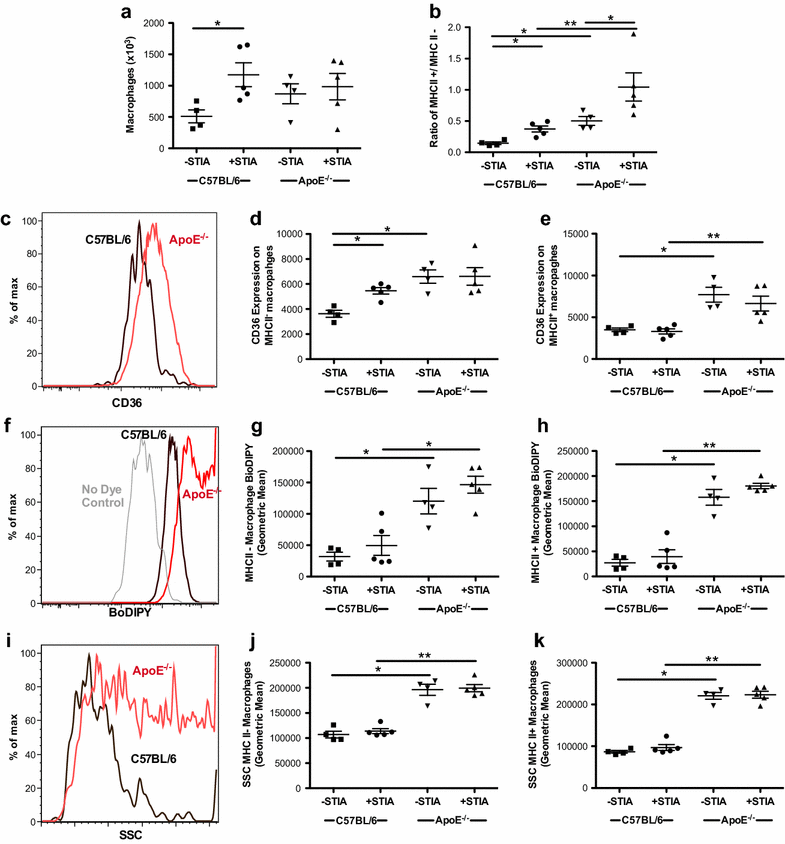
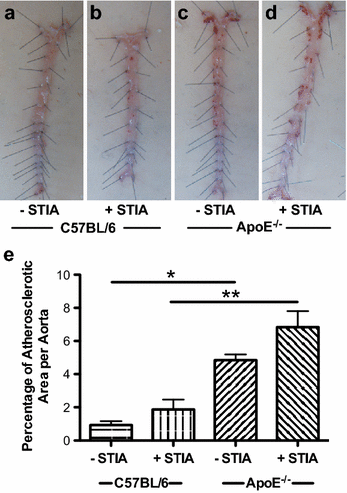
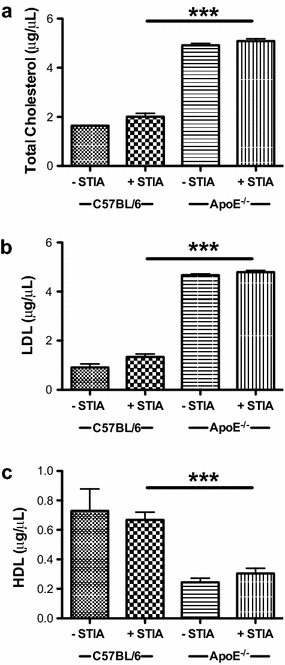
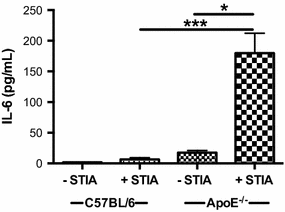
Results: ApoE(-/-) mice developed a sustained and enhanced semi-chronic inflammatory arthritis as compared to control mice. ApoE(-/-) mice had increased numbers of foamy macrophages, enhanced joint inflammation and amplified collagen deposition versus controls. The presence of arthritis did not exacerbate serum cholesterol levels or significantly augment the level of atherosclerosis in ApoE(-/-) mice. However, arthritic ApoE(-/-) mice exhibited a marked elevation of IL-6 as compared to non-arthritic ApoE(-/-) mice and arthritic C57BL/6 mice.
Conclusions: Loss of ApoE potentiates a semi-chronic inflammatory arthritis. This heightened inflammatory response was associated with an increase in circulating IL-6 and in the number of foamy macrophages within the joint. Moreover, the foamy macrophages within the arthritic joint are reminiscent of those within unstable atherosclerotic lesions and suggest a pathologic role for foamy macrophages in propagating arthritis.
Keywords: Animal models of human disease; Arthritis; Cholesterol; Inflammation.
Figures

References
-
- del Rincon I, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-#. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

