GLUT4 Expression in Adipocytes Regulates De Novo Lipogenesis and Levels of a Novel Class of Lipids With Antidiabetic and Anti-inflammatory Effects
- PMID: 27288004
- PMCID: PMC4915575
- DOI: 10.2337/db16-0221
GLUT4 Expression in Adipocytes Regulates De Novo Lipogenesis and Levels of a Novel Class of Lipids With Antidiabetic and Anti-inflammatory Effects
Abstract
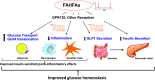
Adipose tissue (AT) regulates systemic insulin sensitivity through multiple mechanisms, and alterations in de novo lipogenesis appear to contribute. Mice overexpressing GLUT4 in adipocytes (AG4OX) have elevated AT lipogenesis and enhanced glucose tolerance despite being obese and having elevated circulating fatty acids. Lipidomic analysis of AT identified a structurally unique class of lipids, branched fatty acid esters of hydroxy-fatty acids (FAHFAs), which were elevated in AT and serum of AG4OX mice. Palmitic acid esters of hydroxy-stearic acids (PAHSAs) are among the most upregulated FAHFA families in AG4OX mice. Eight PAHSA isomers are present in mouse and human tissues. PAHSA levels are reduced in insulin resistant people, and levels correlate highly with insulin sensitivity. PAHSAs have beneficial metabolic effects. Treatment of obese mice with PAHSAs lowers glycemia and improves glucose tolerance while stimulating glucagon-like peptide 1 and insulin secretion. PAHSAs also reduce inflammatory cytokine production from immune cells and ameliorate adipose inflammation in obesity. PAHSA isomer concentrations are altered in physiological and pathophysiological conditions in a tissue- and isomer-specific manner. The mechanisms most likely involve changes in PAHSA biosynthesis, degradation, and secretion. The discovery of PAHSAs reveals the existence of previously unknown endogenous lipids and biochemical pathways involved in metabolism and inflammation, two fundamental physiological processes.
© 2016 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures

Similar articles
-
Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects.Cell. 2014 Oct 9;159(2):318-32. doi: 10.1016/j.cell.2014.09.035. Cell. 2014. PMID: 25303528 Free PMC article.
-
Adipose tissue regulates insulin sensitivity: role of adipogenesis, de novo lipogenesis and novel lipids.J Intern Med. 2016 Nov;280(5):465-475. doi: 10.1111/joim.12540. Epub 2016 Oct 3. J Intern Med. 2016. PMID: 27699898 Free PMC article. Review.
-
Distinct biological activities of isomers from several families of branched fatty acid esters of hydroxy fatty acids (FAHFAs).J Lipid Res. 2021;62:100108. doi: 10.1016/j.jlr.2021.100108. Epub 2021 Aug 18. J Lipid Res. 2021. PMID: 34418413 Free PMC article.
-
Lipokine 5-PAHSA Is Regulated by Adipose Triglyceride Lipase and Primes Adipocytes for De Novo Lipogenesis in Mice.Diabetes. 2020 Mar;69(3):300-312. doi: 10.2337/db19-0494. Epub 2019 Dec 5. Diabetes. 2020. PMID: 31806624 Free PMC article.
-
Omega-3 fatty acids and adipose tissue biology.Mol Aspects Med. 2018 Dec;64:147-160. doi: 10.1016/j.mam.2018.01.004. Epub 2018 Jan 17. Mol Aspects Med. 2018. PMID: 29329795 Review.
Cited by
-
9-PAHSA Improves Cardiovascular Complications by Promoting Autophagic Flux and Reducing Myocardial Hypertrophy in Db/Db Mice.Front Pharmacol. 2021 Nov 15;12:754387. doi: 10.3389/fphar.2021.754387. eCollection 2021. Front Pharmacol. 2021. PMID: 34867366 Free PMC article.
-
Enantioselective Organocatalysis-Based Synthesis of 3-Hydroxy Fatty Acids and Fatty γ-Lactones.Molecules. 2019 May 31;24(11):2081. doi: 10.3390/molecules24112081. Molecules. 2019. PMID: 31159242 Free PMC article.
-
Effects of marine collagen peptides on glucose metabolism and insulin resistance in type 2 diabetic rats.J Food Sci Technol. 2017 Jul;54(8):2260-2269. doi: 10.1007/s13197-017-2663-z. Epub 2017 Jun 14. J Food Sci Technol. 2017. PMID: 28740282 Free PMC article.
-
Bolanthus turcicus: a promising antidiabetic with in-vitro antioxidant, enzyme inhibitory and antiadipogenic activities.J Mol Histol. 2024 Dec 27;56(1):59. doi: 10.1007/s10735-024-10283-5. J Mol Histol. 2024. PMID: 39729235
-
Metformin Monotherapy Alters the Human Plasma Lipidome Independent of Clinical Markers of Glycemic Control and Cardiovascular Disease Risk in a Type 2 Diabetes Clinical Cohort.J Pharmacol Exp Ther. 2023 Aug;386(2):169-180. doi: 10.1124/jpet.122.001493. Epub 2023 Mar 14. J Pharmacol Exp Ther. 2023. PMID: 36918276 Free PMC article.
References
-
- Lifshitz F, Lifshitz JZ. Globesity: the root causes of the obesity epidemic in the USA and now worldwide. Pediatr Endocrinol Rev 2014;12:17–34 - PubMed
-
- Lam DW, LeRoith D. The worldwide diabetes epidemic. Curr Opin Endocrinol Diabetes Obes 2012;19:93–96 - PubMed
-
- Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev 2014;13:981–1000 - PubMed
-
- Procaccini C, Carbone F, Galgani M, et al. . Obesity and susceptibility to autoimmune diseases. Expert Rev Clin Immunol 2011;7:287–294 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

