MAP17 Is a Necessary Activator of Renal Na+/Glucose Cotransporter SGLT2
- PMID: 27288013
- PMCID: PMC5198278
- DOI: 10.1681/ASN.2015111282
MAP17 Is a Necessary Activator of Renal Na+/Glucose Cotransporter SGLT2
Abstract
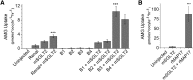
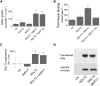
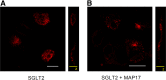
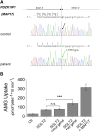
The renal proximal tubule reabsorbs 90% of the filtered glucose load through the Na+-coupled glucose transporter SGLT2, and specific inhibitors of SGLT2 are now available to patients with diabetes to increase urinary glucose excretion. Using expression cloning, we identified an accessory protein, 17 kDa membrane-associated protein (MAP17), that increased SGLT2 activity in RNA-injected Xenopus oocytes by two orders of magnitude. Significant stimulation of SGLT2 activity also occurred in opossum kidney cells cotransfected with SGLT2 and MAP17. Notably, transfection with MAP17 did not change the quantity of SGLT2 protein at the cell surface in either cell type. To confirm the physiologic relevance of the MAP17-SGLT2 interaction, we studied a cohort of 60 individuals with familial renal glucosuria. One patient without any identifiable mutation in the SGLT2 coding gene (SLC5A2) displayed homozygosity for a splicing mutation (c.176+1G>A) in the MAP17 coding gene (PDZK1IP1). In the proximal tubule and in other tissues, MAP17 is known to interact with PDZK1, a scaffolding protein linked to other transporters, including Na+/H+ exchanger 3, and to signaling pathways, such as the A-kinase anchor protein 2/protein kinase A pathway. Thus, these results provide the basis for a more thorough characterization of SGLT2 which would include the possible effects of its inhibition on colocalized renal transporters.
Keywords: Na transport; cell & transport physiology; diabetes; glucose reabsorption; ion transport.
Copyright © 2016 by the American Society of Nephrology.
Figures

References
-
- Sabolic I, Vrhovac I, Eror DB, Gerasimova M, Rose M, Breljak D, Ljubojevic M, Brzica H, Sebastiani A, Thal SC, Sauvant C, Kipp H, Vallon V, Koepsell H: Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol 302: C1174–C1188, 2012 - PMC - PubMed
-
- Wells RG, Pajor AM, Kanai Y, Turk E, Wright EM, Hediger MA: Cloning of a human kidney cDNA with similarity to the sodium-glucose cotransporter. Am J Physiol 263: F459–F465, 1992 - PubMed
-
- Santer R, Kinner M, Schneppenheim R, Hillebrand G, Kemper M, Ehrich J, Swift P, Skovby F, Schaub J: The molecular basis of renal glucosuria: mutations in the gene for a renal glucose transporter (SGLT2). J Inherit Metab Dis 23[Suppl 1]: 178, 2000
-
- Santer R, Kinner M, Lassen CL, Schneppenheim R, Eggert P, Bald M, Brodehl J, Daschner M, Ehrich JH, Kemper M, Li Volti S, Neuhaus T, Skovby F, Swift PG, Schaub J, Klaerke D: Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol 14: 2873–2882, 2003 - PubMed
-
- Ullman CG, Frigotto L, Cooley RN: In vitro methods for peptide display and their applications. Brief Funct Genomics 10: 125–134, 2011 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

