The Long and Winding Road: From the High-Affinity Choline Uptake Site to Clinical Trials for Malignant Brain Tumors
- PMID: 27288077
- PMCID: PMC4997808
- DOI: 10.1016/bs.apha.2016.03.002
The Long and Winding Road: From the High-Affinity Choline Uptake Site to Clinical Trials for Malignant Brain Tumors
Abstract
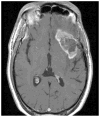
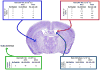
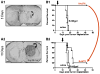
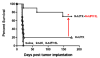
Malignant brain tumors are one of the most lethal cancers. They originate from glial cells which infiltrate throughout the brain. Current standard of care involves surgical resection, radiotherapy, and chemotherapy; median survival is currently ~14-20 months postdiagnosis. Given that the brain immune system is deficient in priming systemic immune responses to glioma antigens, we proposed to reconstitute the brain immune system to achieve immunological priming from within the brain. Two adenoviral vectors are injected into the resection cavity or remaining tumor. One adenoviral vector expresses the HSV-1-derived thymidine kinase which converts ganciclovir into a compound only cytotoxic to dividing glioma cells. The second adenovirus expresses the cytokine fms-like tyrosine kinase 3 ligand (Flt3L). Flt3L differentiates precursors into dendritic cells and acts as a chemokine that attracts dendritic cells to the brain. HSV-1/ganciclovir killing of tumor cells releases tumor antigens that are taken up by dendritic cells within the brain tumor microenvironment. Tumor killing also releases HMGB1, an endogenous TLR2 agonist that activates dendritic cells. HMGB1-activated dendritic cells, loaded with glioma antigens, migrate to cervical lymph nodes to stimulate a systemic CD8+ T cells cytotoxic immune response against glioma. This immune response is specific to glioma tumors, induces immunological memory, and does neither cause brain toxicity nor autoimmune responses. An IND was granted by the FDA on 4/7/2011. A Phase I, first in person trial, to test whether reengineering the brain immune system is potentially therapeutic is ongoing.
Keywords: Brain tumors; Cancer; Flt3L; Gene therapy; Immunotherapy.
© 2016 Elsevier Inc. All rights reserved.
Figures


References
-
- Abordo-Adesida E, Follenzi A, Barcia C, Sciascia S, Castro MG, Naldini L, Lowenstein PR. Stability of lentiviral vector-mediated transgene expression in the brain in the presence of systemic antivector immune responses. Hum Gene Ther. 2005;16(6):741–751. doi: 10.1089/hum.2005.16.741. - DOI - PMC - PubMed
-
- Ali S, Curtin JF, Zirger JM, Xiong W, King GD, Barcia C, Castro MG. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10(6):1071–1084. doi: 10.1016/j.ymthe.2004.08.025. S1525-0016(04)01422-4 [pii] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

