From Neuron Biophysics to Orientation Selectivity in Electrically Coupled Networks of Neocortical L2/3 Large Basket Cells
- PMID: 27288316
- PMCID: PMC4961030
- DOI: 10.1093/cercor/bhw166
From Neuron Biophysics to Orientation Selectivity in Electrically Coupled Networks of Neocortical L2/3 Large Basket Cells
Abstract
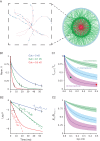
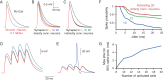
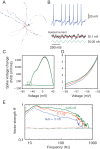
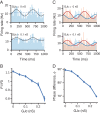
In the neocortex, inhibitory interneurons of the same subtype are electrically coupled with each other via dendritic gap junctions (GJs). The impact of multiple GJs on the biophysical properties of interneurons and thus on their input processing is unclear. The present experimentally based theoretical study examined GJs in L2/3 large basket cells (L2/3 LBCs) with 3 goals in mind: (1) To evaluate the errors due to GJs in estimating the cable properties of individual L2/3 LBCs and suggest ways to correct these errors when modeling these cells and the networks they form; (2) to bracket the GJ conductance value (0.05-0.25 nS) and membrane resistivity (10 000-40 000 Ω cm(2)) of L2/3 LBCs; these estimates are tightly constrained by in vitro input resistance (131 ± 18.5 MΩ) and the coupling coefficient (1-3.5%) of these cells; and (3) to explore the functional implications of GJs, and show that GJs: (i) dynamically modulate the effective time window for synaptic integration; (ii) improve the axon's capability to encode rapid changes in synaptic inputs; and (iii) reduce the orientation selectivity, linearity index, and phase difference of L2/3 LBCs. Our study provides new insights into the role of GJs and calls for caution when using in vitro measurements for modeling electrically coupled neuronal networks.
Keywords: cortical interneurons; electrical coupling; gap junctions; membrane time constant; visual cortex.
© The Author 2016. Published by Oxford University Press.
Figures




References
-
- Angulo MC, Rossier J, Audinat E. 1999. Postsynaptic glutamate receptors and integrative properties of fast-spiking interneurons in the rat neocortex. J Neurophysiol. 82:1295–1302. - PubMed
-
- Avermann M, Tomm C, Mateo C, Gerstner W, Petersen CCH. 2012. Microcircuits of excitatory and inhibitory neurons in layer 2/3 of mouse barrel cortex. J Neurophysiol. 107:3116–3134. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

