Protocol for a pilot randomised controlled trial of an online intervention for post-treatment cancer survivors with persistent fatigue
- PMID: 27288384
- PMCID: PMC4908920
- DOI: 10.1136/bmjopen-2016-011485
Protocol for a pilot randomised controlled trial of an online intervention for post-treatment cancer survivors with persistent fatigue
Abstract
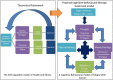
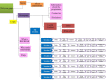
Introduction: Many post-treatment cancer survivors experience persistent fatigue that can disrupt attempts to resume normal everyday activities after treatment. Theoretical models that aim to explain contributory factors that initiate and sustain fatigue symptoms, or that influence the efficacy of interventions for cancer-related fatigue (CrF) require testing. Adjustment to fatigue is likely to be influenced by coping behaviours that are guided by the representations of the symptom.
Objectives: This paper describes the protocol for a pilot trial of a systematically and theoretically designed online intervention to enable self-management of CrF after cancer treatment.
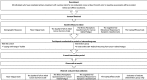
Methods and analysis: This 2-armed randomised controlled pilot trial will study the feasibility and potential effectiveness of an online intervention. Participants will be allocated to either the online intervention (REFRESH (Recovery from Cancer-Related Fatigue)), or a leaflet comparator.
Participants: 80 post-treatment cancer survivors will be recruited for the study.
Interventions: An 8-week online intervention based on cognitive-behavioural therapy.
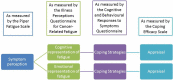
Primary and secondary outcome measures: The primary outcome is a change in fatigue as measured by the Piper Fatigue Scale (revised). Quality of life will be measured using the Quality of Life in Adult Survivors of Cancer Scale. Outcome measures will be collected at baseline, and at completion of intervention.
Results: The feasibility of trial procedures will be tested, as well as the effect of the intervention on the outcomes.
Conclusions: This study may lead to the development of a supportive resource to target representations and coping strategies of cancer survivors with CrF post-treatment.
Setting: Recruitment from general public in Ireland.
Ethics and dissemination: This trial was approved by the Research Ethics Committee at National University of Ireland Galway in January 2013. Trial results will be communicated in a peer-reviewed journal.
Trial registration number: ISRCTN55763085; Pre-results.
Keywords: Cancer survivors; fatigue; self-regulation model.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

Similar articles
-
RESTORE: an exploratory trial of an online intervention to enhance self-efficacy to manage problems associated with cancer-related fatigue following primary cancer treatment: study protocol for a randomized controlled trial.Trials. 2013 Jun 21;14:184. doi: 10.1186/1745-6215-14-184. Trials. 2013. PMID: 23786716 Free PMC article. Clinical Trial.
-
Telehealth cancer-related fatigue clinic model for cancer survivors: a pilot randomised controlled trial protocol (the T-CRF trial).BMJ Open. 2022 May 16;12(5):e059952. doi: 10.1136/bmjopen-2021-059952. BMJ Open. 2022. PMID: 35577469 Free PMC article.
-
Guided internet-administered self-help to reduce symptoms of anxiety and depression among adolescents and young adults diagnosed with cancer during adolescence (U-CARE: YoungCan): a study protocol for a feasibility trial.BMJ Open. 2017 Jan 27;7(1):e013906. doi: 10.1136/bmjopen-2016-013906. BMJ Open. 2017. PMID: 28132011 Free PMC article.
-
The effectiveness of psychological interventions for fatigue in cancer survivors: systematic review of randomised controlled trials.Syst Rev. 2019 Dec 13;8(1):324. doi: 10.1186/s13643-019-1230-2. Syst Rev. 2019. PMID: 31836007 Free PMC article.
-
Management of side effects during and post-treatment in breast cancer survivors.Breast J. 2018 Mar;24(2):167-175. doi: 10.1111/tbj.12862. Epub 2017 Aug 27. Breast J. 2018. PMID: 28845551 Review.
Cited by
-
Cancer-Related Fatigue in Post-Treatment Cancer Survivors: Theory-Based Development of a Web-Based Intervention.JMIR Cancer. 2017 Jul 4;3(2):e8. doi: 10.2196/cancer.6987. JMIR Cancer. 2017. PMID: 28676465 Free PMC article.
-
Efficacy of a Combined Acceptance and Commitment Intervention to Improve Psychological Flexibility and Associated Symptoms in Cancer Patients: Study Protocol for a Randomized Controlled Trial.Front Psychol. 2022 May 18;13:871929. doi: 10.3389/fpsyg.2022.871929. eCollection 2022. Front Psychol. 2022. PMID: 35664159 Free PMC article.
-
Therapeutic Education and Physical Activity to Support Self-management of Cancer-related Fatigue in Hematologic Cancer Patients: Protocol of a Feasibility Randomized Controlled Trial.Integr Cancer Ther. 2020 Jan-Dec;19:1534735420969830. doi: 10.1177/1534735420969830. Integr Cancer Ther. 2020. PMID: 33243016 Free PMC article.
-
Educational interventions for the management of cancer-related fatigue in adults.Cochrane Database Syst Rev. 2016 Nov 24;11(11):CD008144. doi: 10.1002/14651858.CD008144.pub2. Cochrane Database Syst Rev. 2016. PMID: 27883365 Free PMC article.
-
Patient preference attributes in eHealth interventions for cancer-related fatigue: A scoping review.Eur J Cancer Care (Engl). 2022 Nov;31(6):e13754. doi: 10.1111/ecc.13754. Epub 2022 Nov 16. Eur J Cancer Care (Engl). 2022. PMID: 36385440 Free PMC article.
References
-
- Aziz NM, Rowland JH, eds. Trends and advances in cancer survivorship research: challenge and opportunity. Seminars in radiation oncology. Elsevier, 2003. - PubMed
-
- Mock V, Atkinson A, Barsevick A et al. . NCCN practice guidelines for cancer-related fatigue. Oncology (Williston Park) 2000;14:151–61. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
