Gut microbiota are linked to increased susceptibility to hepatic steatosis in low-aerobic-capacity rats fed an acute high-fat diet
- PMID: 27288420
- PMCID: PMC4967176
- DOI: 10.1152/ajpgi.00065.2016
Gut microbiota are linked to increased susceptibility to hepatic steatosis in low-aerobic-capacity rats fed an acute high-fat diet
Abstract
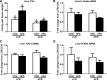
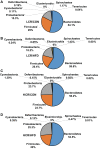
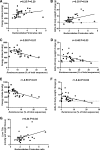
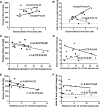
Poor aerobic fitness is linked to nonalcoholic fatty liver disease and increased all-cause mortality. We previously found that rats with a low capacity for running (LCR) that were fed an acute high-fat diet (HFD; 45% kcal from fat) for 3 days resulted in positive energy balance and increased hepatic steatosis compared with rats that were highly aerobically fit with a high capacity for running (HCR). Here, we tested the hypothesis that poor physiological outcomes in LCR rats following acute HFD feeding are associated with alterations in cecal microbiota. LCR rats exhibited greater body weight, feeding efficiency, 3 days of body weight change, and liver triglycerides after acute HFD feeding compared with HCR rats. Furthermore, compared with HCR rats, LCR rats exhibited reduced expression of intestinal tight junction proteins. Cecal bacterial 16S rDNA revealed that LCR rats had reduced cecal Proteobacteria compared with HCR rats. Microbiota of HCR rats consisted of greater relative abundance of Desulfovibrionaceae and unassigned genera within this family, suggesting increased reduction of endogenous mucins and proteins. Although feeding rats an acute HFD led to reduced Firmicutes in both strains, short-chain fatty acid-producing Phascolarctobacterium was reduced in LCR rats. In addition, Ruminococcae and Ruminococcus were negatively correlated with energy intake in the LCR/HFD rats. Predicted metagenomic function suggested that LCR rats had a greater capacity to metabolize carbohydrate and energy compared with HCR rats. Overall, these data suggest that the populations and metabolic capacity of the microbiota in low-aerobically fit LCR rats may contribute to their susceptibility to acute HFD-induced hepatic steatosis and poor physiologic outcomes.
Keywords: NAFLD; aerobic fitness; microbiota; short-chain fatty acids.
Figures





Similar articles
-
Aerobic Capacity and Exercise Mediate Protection Against Hepatic Steatosis via Enhanced Bile Acid Metabolism.Function (Oxf). 2025 May 19;6(3):zqaf019. doi: 10.1093/function/zqaf019. Function (Oxf). 2025. PMID: 40194946 Free PMC article.
-
Aerobic capacity and hepatic mitochondrial lipid oxidation alters susceptibility for chronic high-fat diet-induced hepatic steatosis.Am J Physiol Endocrinol Metab. 2016 Oct 1;311(4):E749-E760. doi: 10.1152/ajpendo.00178.2016. Epub 2016 Sep 6. Am J Physiol Endocrinol Metab. 2016. PMID: 27600823 Free PMC article.
-
Shared and distinct adaptations to early-life exercise training based on inborn fitness.J Physiol. 2025 Jun 15. doi: 10.1113/JP288331. Online ahead of print. J Physiol. 2025. PMID: 40517393
-
Revisiting the concepts of de novo lipogenesis to understand the conversion of carbohydrates into fats: Stop overvaluing and extrapolating the renowned phrase "fat burns in the flame of carbohydrate".Nutrition. 2025 Feb;130:112617. doi: 10.1016/j.nut.2024.112617. Epub 2024 Oct 24. Nutrition. 2025. PMID: 39566326 Review.
-
Gut microbiota and its derived metabolites as silent sculptors of non-alcoholic fatty liver disease development and potential guide for non-invasive clinical diagnostics.Mol Biol Rep. 2025 Aug 7;52(1):800. doi: 10.1007/s11033-025-10901-9. Mol Biol Rep. 2025. PMID: 40775101 Review.
Cited by
-
Altered Intestinal Production of Volatile Fatty Acids in Dogs Triggered by Lactulose and Psyllium Treatment.Vet Sci. 2022 Apr 23;9(5):206. doi: 10.3390/vetsci9050206. Vet Sci. 2022. PMID: 35622734 Free PMC article.
-
Vertical selection for nuclear and mitochondrial genomes shapes gut microbiota and modifies risks for complex diseases.Physiol Genomics. 2020 Jan 1;52(1):1-14. doi: 10.1152/physiolgenomics.00089.2019. Epub 2019 Nov 25. Physiol Genomics. 2020. PMID: 31762410 Free PMC article.
-
Gut microbiota is correlated with gastrointestinal adverse events of metformin in patients with type 2 diabetes.Front Endocrinol (Lausanne). 2022 Nov 17;13:1044030. doi: 10.3389/fendo.2022.1044030. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36465607 Free PMC article.
-
Research Note: Increase of bad bacteria and decrease of good bacteria in the gut of layers with vs. without hepatic steatosis.Poult Sci. 2020 Oct;99(10):5074-5078. doi: 10.1016/j.psj.2020.07.007. Epub 2020 Aug 5. Poult Sci. 2020. PMID: 32988545 Free PMC article.
-
In Vitro Hepatoprotective and Human Gut Microbiota Modulation of Polysaccharide-Peptides in Pleurotus citrinopileatus.Front Cell Infect Microbiol. 2022 May 20;12:892049. doi: 10.3389/fcimb.2022.892049. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35669115 Free PMC article.
References
-
- Allen JM, Berg Miller ME, Pence BD, Whitlock K, Nehra V, Gaskins HR, White BA, Fryer JD, Woods JA. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J Appl Physiol 118: 1059–1066, 2015. - PubMed
-
- Bermon S, Petriz B, Kajeniene A, Prestes J, Castell L, Franco O. The microbiota: an exercise immunology perspective. Exerc Immunol Rev 21: 70–79, 2015. - PubMed
-
- Biddle A, Stewart L, Blanchard J, Leschine S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity 5: 627–640, 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

