Angiocrine signaling in the hepatic sinusoids in health and disease
- PMID: 27288423
- PMCID: PMC5007289
- DOI: 10.1152/ajpgi.00118.2016
Angiocrine signaling in the hepatic sinusoids in health and disease
Abstract
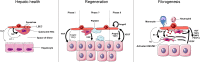
The capillary network irrigating the liver is important not only for nutrient and oxygen delivery, but also for the signals distributed to other hepatic cell types necessary to maintain liver homeostasis. During development, endothelial cells are a key component in liver zonation. In adulthood, they maintain hepatic stellate cells and hepatocytes in quiescence. Their importance in pathobiology is highlighted in liver regeneration and chronic liver diseases, where they coordinate paracrine cell behavior. During regeneration, liver sinusoidal endothelial cells induce hepatocyte proliferation and angiogenesis. During fibrogenesis, they undergo morphological and functional changes, which are reflected by their role in hepatic stellate cell activation, inflammation, and distorted sinusoidal structure. Therapeutic strategies to target angiocrine signaling are in progress but are in the early stages. Here, we offer a short synthesis of recent studies on angiocrine signaling in liver homeostasis, regeneration, and fibrogenesis.
Keywords: angiocrine signaling; angiogenesis; endothelial cell; fibrosis; liver regeneration.
Copyright © 2016 the American Physiological Society.
Figures
Similar articles
-
Angiocrine signaling in sinusoidal homeostasis and liver diseases.J Hepatol. 2024 Sep;81(3):543-561. doi: 10.1016/j.jhep.2024.05.014. Epub 2024 May 17. J Hepatol. 2024. PMID: 38763358 Review.
-
Interaction of non‑parenchymal hepatocytes in the process of hepatic fibrosis (Review).Mol Med Rep. 2021 May;23(5):364. doi: 10.3892/mmr.2021.12003. Epub 2021 Mar 24. Mol Med Rep. 2021. PMID: 33760176 Free PMC article. Review.
-
Endothelial GATA4 controls liver fibrosis and regeneration by preventing a pathogenic switch in angiocrine signaling.J Hepatol. 2021 Feb;74(2):380-393. doi: 10.1016/j.jhep.2020.08.033. Epub 2020 Sep 9. J Hepatol. 2021. PMID: 32916216
-
Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis.Nature. 2014 Jan 2;505(7481):97-102. doi: 10.1038/nature12681. Epub 2013 Nov 20. Nature. 2014. PMID: 24256728 Free PMC article.
-
Angiocrine Bmp2 signaling in murine liver controls normal iron homeostasis.Blood. 2017 Jan 26;129(4):415-419. doi: 10.1182/blood-2016-07-729822. Epub 2016 Nov 30. Blood. 2017. PMID: 27903529 Free PMC article.
Cited by
-
Lyve-1 deficiency enhances the hepatic immune microenvironment entailing altered susceptibility to melanoma liver metastasis.Cancer Cell Int. 2022 Dec 10;22(1):398. doi: 10.1186/s12935-022-02800-x. Cancer Cell Int. 2022. PMID: 36496412 Free PMC article.
-
Plumbagin Alleviates Capillarization of Hepatic Sinusoids In Vitro by Downregulating ET-1, VEGF, LN, and Type IV Collagen.Biomed Res Int. 2017;2017:5603216. doi: 10.1155/2017/5603216. Epub 2017 Jul 9. Biomed Res Int. 2017. PMID: 28770223 Free PMC article.
-
Endothelial Slc35a1 Deficiency Causes Loss of LSEC Identity and Exacerbates Neonatal Lipid Deposition in the Liver in Mice.Cell Mol Gastroenterol Hepatol. 2024;17(6):1039-1061. doi: 10.1016/j.jcmgh.2024.03.002. Epub 2024 Mar 11. Cell Mol Gastroenterol Hepatol. 2024. PMID: 38467191 Free PMC article.
-
Angiocrine signaling in sinusoidal homeostasis and liver diseases.J Hepatol. 2024 Sep;81(3):543-561. doi: 10.1016/j.jhep.2024.05.014. Epub 2024 May 17. J Hepatol. 2024. PMID: 38763358 Review.
-
Angiodiversity and organotypic functions of sinusoidal endothelial cells.Angiogenesis. 2021 May;24(2):289-310. doi: 10.1007/s10456-021-09780-y. Epub 2021 Mar 21. Angiogenesis. 2021. PMID: 33745018 Free PMC article. Review.
References
-
- Adams DH, Eksteen B. Aberrant homing of mucosal T cells and extra-intestinal manifestations of inflammatory bowel disease. Nat Rev Immunol 6: 244–251, 2006. - PubMed
-
- Brunt EM, Gouw AS, Hubscher SG, Tiniakos DG, Bedossa P, Burt AD, Callea F, Clouston AD, Dienes HP, Goodman ZD, Roberts EA, Roskams T, Terracciano L, Torbenson MS, Wanless IR. Pathology of the liver sinusoids. Histopathology 64: 907–920, 2014. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

