Delayed Hypoxemia Following Traumatic Brain Injury Exacerbates White Matter Injury
- PMID: 27288907
- PMCID: PMC7299434
- DOI: 10.1093/jnen/nlw045
Delayed Hypoxemia Following Traumatic Brain Injury Exacerbates White Matter Injury
Abstract
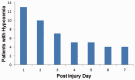
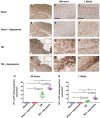
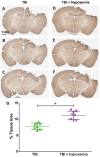
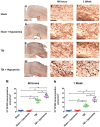
Hypoxemia immediately following traumatic brain injury (TBI) has been observed to exacerbate injury. However, it remains unclear whether delayed hypoxemia beyond the immediate postinjury period influences white matter injury. In a retrospective clinical cohort of children aged 4-16 years admitted with severe TBI, 28/74 (35%) patients were found to experience delayed normocarbic hypoxemia within 7 days of admission. Based on these clinical findings, we developed a clinically relevant mouse model of TBI with delayed hypoxemia by exposing 5-week old (adolescent) mice to hypoxic conditions for 30 minutes starting 24 hours after moderate controlled cortical impact (CCI). Injured mice with hypoxemia had increased axonal injury using both β-amyloid precursor protein and NF200 immunostaining in peri-contusional white matter compared with CCI alone. Furthermore, we detected increased peri-contusional white matter tissue hypoxia with pimonidazole and augmented astrogliosis with anti-glial fibrillary acidic protein staining in CCI + delayed hypoxemia compared with CCI alone or sham surgery + delayed hypoxemia. Microglial activation as evidenced by Iba1 staining was not significantly altered by delayed hypoxemia. These clinical and experimental data indicate the prevention or amelioration of delayed hypoxemia effects following TBI may provide a unique opportunity for the development of therapeutic interventions to reduce axonal injury and improve clinical outcomes.
Keywords: Axonal injury; Brain hypoxia; Controlled cortical impact; Delayed hypoxemia; Secondary injury; Traumatic brain injury.
© 2016 American Association of Neuropathologists, Inc. All rights reserved.
Figures






References
-
- Heron M, Sutton PD, Xu J , et al. . Annual summary of vital statistics: 2007 . Pediatrics 2010. ; 125 : 4 – 15 - PubMed
-
- Fisher MD. Pediatric traumatic brain injury . Crit Care Nurs 1997. ; 20 : 36 – 51 - PubMed
-
- Kochanek PM, Clark RS, Ruppel RA , et al. . Biochemical, cellular, and molecular mechanisms in the evolution of secondary damage after severe traumatic brain injury in infants and children: Lessons learned from the bedside . Pediatr Crit Care Med 2000. ; 1 : 4 – 19 - PubMed
-
- Chesnut RM, Marshall LF, Klauber MR , et al. . The role of secondary brain injury in determining outcome from severe head injury . J Trauma 1993. ; 34 : 216 – 22 - PubMed
-
- Chi JH, Knudson MM, Vassar MJ , et al. . Prehospital hypoxia affects outcome in patients with traumatic brain injury: A prospective multicenter study . J Trauma 2006. ; 61 : 1134 – 41 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

