Persistent Effects of Intensive Glycemic Control on Retinopathy in Type 2 Diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Follow-On Study
- PMID: 27289122
- PMCID: PMC4915557
- DOI: 10.2337/dc16-0024
Persistent Effects of Intensive Glycemic Control on Retinopathy in Type 2 Diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Follow-On Study
Abstract
Objectives: This study investigated whether the beneficial effects of intensive glycemic control and fenofibrate treatment of dyslipidemia in reducing retinopathy progression demonstrated in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study persisted beyond the clinical trial.
Research design and methods: The ACCORD Study (2003-2009) randomized participants with type 2 diabetes to intensive or standard treatment for glycemia (A1C level at <6.0% [42 mmol/mol] vs. 7.0-7.9% [53-63 mmol/mol]), systolic blood pressure (<120 vs. 140 mmHg), and dyslipidemia (fenofibrate [160 mg] plus simvastatin or placebo plus simvastatin). ACCORD Eye Study participants, who had baseline and year 4 eye examinations and fundus photographs, were reexamined in the ACCORD Follow-On (ACCORDION) Eye Study (2010-2014) 4 years after the ACCORD trial closeout. The outcome measure was diabetic retinopathy progression of three or more steps on the Early Treatment Diabetic Retinopathy Study scale.
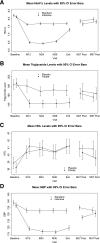
Results: Diabetic retinopathy progressed in 5.8% with intensive glycemic treatment versus 12.7% with standard (adjusted odds ratio [aOR] 0.42, 95% CI 0.28-0.63, P < 0.0001), 7.5% with intensive blood pressure treatment versus 6.0% for standard (aOR 1.21, 95% CI 0.61-2.40, P = 0.59), and 11.8% with fenofibrate versus 10.2% with placebo (aOR 1.13, 95% CI 0.71-1.79, P = 0.60) in ACCORDION Eye participants (n = 1,310).
Conclusions: Prior intensive glycemic control continued to reduce diabetic retinopathy progression, despite similar A1C levels, when the ACCORD Study ended. This is the first study in people with type 2 diabetes of 10 years' duration and established cardiovascular disease, unlike the newly diagnosed participants of the UK Prospective Diabetes Study, to demonstrate this effect. The benefit of fenofibrate, however, did not persist. Intensive blood pressure control had no effect.
Trial registration: ClinicalTrials.gov NCT00000620 NCT00542178.
© 2016 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Figures



References
-
- UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 - PubMed
-
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 - PubMed
-
- The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 - PubMed
-
- Keech AC, Mitchell P, Summanen PA, et al. ; FIELD study investigators . Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 2007;370:1687–1697 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

