The effect of prenatal docosahexaenoic acid supplementation on infant outcomes in African American women living in low-income environments: A randomized, controlled trial
- PMID: 27290652
- PMCID: PMC4955755
- DOI: 10.1016/j.psyneuen.2016.05.023
The effect of prenatal docosahexaenoic acid supplementation on infant outcomes in African American women living in low-income environments: A randomized, controlled trial
Abstract
Importance: African American women living in urban, low-income environments are at high risk for poor nutrition during pregnancy and birth complications.
Objective: To test the effectiveness of prenatal docosahexaenoic acid (DHA) supplementation on birth outcomes and infant development in a sample of African American women with Medicaid insurance and living in the city of Pittsburgh.
Design: The Nutrition and Pregnancy Study (NAPS) is a double-blind, randomized controlled trial of prenatal DHA supplementation conducted between 2012 and 2014.
Setting: Participants were recruited from obstetric clinics at the University of Pittsburgh Medical Center.
Participants: Sixty-four pregnant, African American women were enrolled at 16-21 weeks of gestation and randomized to either 450mg/day of DHA (22:6n-3)(n=43) or a soybean placebo (n=21). Four women (6.3%) withdrew from the study: two participants from each study arm; complete data were obtained for 49 infants (76.5%) at the 3-month assessment.
Interventions: Supplementation with DHA or placebo continued from the beginning of enrollment through delivery.
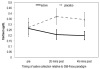
Main outcome and measures: Data on birth outcomes were collected from medical records. At approximately 3 months post-partum, mothers brought their infants to the laboratory where the Bayley Scales of Infant Development (BSID-III) were administered and cortisol response to the Face-to-Face Still-Face (FFSF) paradigm was assessed.
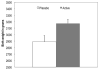
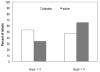
Results: Infants of mothers who received DHA supplementation had higher birth weight (3.174g versus 2.890g) than infants of mothers receiving placebo (F [2.40]=6.09, p=0.018, eta=0.36), and were more likely to have a 1-min Apgar score greater than 8 (OR=5.99 [95% CI=1.25-28.75], p=0.025). Infants of mothers who received DHA compared with infants of mothers receiving placebo had lower levels of cortisol in response to the FFSF paradigm (F [1.32]=5.36, p=0.018, eta=0.36). None of the scores on the BSID-III differed as a function of active supplement versus placebo.
Conclusions: Infants of women living in urban, low-income environments who received DHA supplementation had more optimal birth outcomes and more modulated cortisol response to a stressor. DHA supplementation may be effective in attenuating the negative effects of prenatal stress on offspring development.
Keywords: Birth outcomes; Cortisol; Docosahexaenoic acid; Infant; Prenatal stress; Supplementation.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
None of the authors report conflicts of interest with the data presented in this paper
Figures

References
-
- American College of Obstetricians and Gynecologists. The Apgar score. Committee Opinion No 644. Obstet Gynecol. 2015;126:e52–5. - PubMed
-
- Bayley N. Bayley Scales of Infant and Toddler Development. 3. San Antonio, TX: Harcourt Assessment Inc; 2006.
-
- DeNavas-Walt C, Proctor BD. Income and Poverty in the United States: 2014. U.S. Government Printing Office; Washington, DC: 2015. U.S. Census Bureau, Current Population Reports, P60–252.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

