Revisiting Frank-Starling: regulatory light chain phosphorylation alters the rate of force redevelopment (ktr ) in a length-dependent fashion
- PMID: 27291932
- PMCID: PMC5023691
- DOI: 10.1113/JP272441
Revisiting Frank-Starling: regulatory light chain phosphorylation alters the rate of force redevelopment (ktr ) in a length-dependent fashion
Abstract
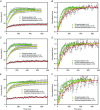
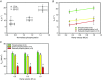
Key points: Regulatory light chain (RLC) phosphorylation has been shown to alter the ability of muscle to produce force and power during shortening and to alter the rate of force redevelopment (ktr ) at submaximal [Ca(2+) ]. Increasing RLC phosphorylation ∼50% from the in vivo level in maximally [Ca(2+) ]-activated cardiac trabecula accelerates ktr . Decreasing RLC phosphorylation to ∼70% of the in vivo control level slows ktr and reduces force generation. ktr is dependent on sarcomere length in the physiological range 1.85-1.94 μm and RLC phosphorylation modulates this response. We demonstrate that Frank-Starling is evident at maximal [Ca(2+) ] activation and therefore does not necessarily require length-dependent change in [Ca(2+) ]-sensitivity of thin filament activation. The stretch response is modulated by changes in RLC phosphorylation, pinpointing RLC phosphorylation as a modulator of the Frank-Starling law in the heart. These data provide an explanation for slowed systolic function in the intact heart in response to RLC phosphorylation reduction.
Abstract: Force and power in cardiac muscle have a known dependence on phosphorylation of the myosin-associated regulatory light chain (RLC). We explore the effect of RLC phosphorylation on the ability of cardiac preparations to redevelop force (ktr ) in maximally activating [Ca(2+) ]. Activation was achieved by rapidly increasing the temperature (temperature-jump of 0.5-20ºC) of permeabilized trabeculae over a physiological range of sarcomere lengths (1.85-1.94 μm). The trabeculae were subjected to shortening ramps over a range of velocities and the extent of RLC phosphorylation was varied. The latter was achieved using an RLC-exchange technique, which avoids changes in the phosphorylation level of other proteins. The results show that increasing RLC phosphorylation by 50% accelerates ktr by ∼50%, irrespective of the sarcomere length, whereas decreasing phosphorylation by 30% slows ktr by ∼50%, relative to the ktr obtained for in vivo phosphorylation. Clearly, phosphorylation affects the magnitude of ktr following step shortening or ramp shortening. Using a two-state model, we explore the effect of RLC phosphorylation on the kinetics of force development, which proposes that phosphorylation affects the kinetics of both attachment and detachment of cross-bridges. In summary, RLC phosphorylation affects the rate and extent of force redevelopment. These findings were obtained in maximally activated muscle at saturating [Ca(2+) ] and are not explained by changes in the Ca(2+) -sensitivity of acto-myosin interactions. The length-dependence of the rate of force redevelopment, together with the modulation by the state of RLC phosphorylation, suggests that these effects play a role in the Frank-Starling law of the heart.
Keywords: cardiac muscle; force redevelopment; muscle contraction; phosphorylation; regulatory light chain.
© 2016 Wellcome Trust The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Figures




References
-
- Aoki H, Sadoshima J & Izumo S (2000). Myosin light chain kinase mediates sarcomere organization during cardiac hypertrophy in vitro. Nat Med 6, 183–188. - PubMed
-
- Araujo A & Walker JW (1994). Kinetics of tension development in skinned cardiac myocytes measured by photorelease of Ca2+ . Am J Physiol Heart Circ Physiol 267, H1643–H1653. - PubMed
-
- Barany K, Barany M, Gillis JM & Kushmerick MJ (1980). Myosin light chain phosphorylation during the contraction cycle of frog muscle. Fed Proc 39, 1547–1551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

