Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities
- PMID: 27292029
- PMCID: PMC4910019
- DOI: 10.1038/ncomms11852
Long-lived self-renewing bone marrow-derived macrophages displace embryo-derived cells to inhabit adult serous cavities
Abstract
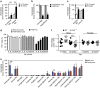
Peritoneal macrophages are one of the most studied macrophage populations in the body, yet the composition, developmental origin and mechanisms governing the maintenance of this compartment are controversial. Here we show resident F4/80(hi)GATA6(+) macrophages are long-lived, undergo non-stochastic self-renewal and retain cells of embryonic origin for at least 4 months in mice. However, Ly6C(+) monocytes constitutively enter the peritoneal cavity in a CCR2-dependent manner, where they mature into short-lived F4/80(lo)MHCII(+) cells that act, in part, as precursors of F4/80(hi)GATA6(+) macrophages. Notably, monocyte-derived F4/80(hi) macrophages eventually displace the embryonic population with age in a process that is highly gender dependent and not due to proliferative exhaustion of the incumbent embryonic population, despite the greater proliferative activity of newly recruited cells. Furthermore, although monocyte-derived cells acquire key characteristics of the embryonic population, expression of Tim4 was impaired, leading to cumulative changes in the population with age.
Figures





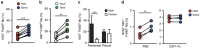
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

