CD40 Stimulation Obviates Innate Sensors and Drives T Cell Immunity in Cancer
- PMID: 27292635
- PMCID: PMC4917417
- DOI: 10.1016/j.celrep.2016.05.058
CD40 Stimulation Obviates Innate Sensors and Drives T Cell Immunity in Cancer
Abstract
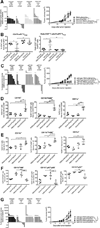
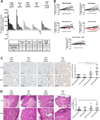
Cancer immunotherapies are more effective in tumors with robust T cell infiltrates, but mechanisms to convert T cell-devoid tumors with active immunosuppression to those capable of recruiting T cells remain incompletely understood. Here, using genetically engineered mouse models of pancreatic ductal adenocarcinoma (PDA), we demonstrate that a single dose of agonistic CD40 antibody with chemotherapy rendered PDA susceptible to T cell-dependent destruction and potentiated durable remissions. CD40 stimulation caused a clonal expansion of T cells in the tumor, but the addition of chemotherapy optimized myeloid activation and T cell function. Although recent data highlight the requirement for innate sensors in cancer immunity, these canonical pathways-including TLRs, inflammasome, and type I interferon/STING-played no role in mediating the efficacy of CD40 and chemotherapy. Thus, CD40 functions as a non-redundant mechanism to convert the tumor microenvironment immunologically. Our data provide a rationale for a newly initiated clinical trial of CD40 and chemotherapy in PDA.
Copyright © 2016 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13:1050–1059. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

