Discovery of an Active RAG Transposon Illuminates the Origins of V(D)J Recombination
- PMID: 27293192
- PMCID: PMC5017859
- DOI: 10.1016/j.cell.2016.05.032
Discovery of an Active RAG Transposon Illuminates the Origins of V(D)J Recombination
Abstract
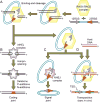
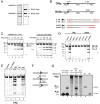
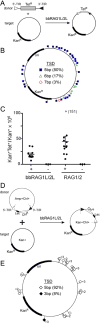
Co-option of RAG1 and RAG2 for antigen receptor gene assembly by V(D)J recombination was a crucial event in the evolution of jawed vertebrate adaptive immunity. RAG1/2 are proposed to have arisen from a transposable element, but definitive evidence for this is lacking. Here, we report the discovery of ProtoRAG, a DNA transposon family from lancelets, the most basal extant chordates. A typical ProtoRAG is flanked by 5-bp target site duplications and a pair of terminal inverted repeats (TIRs) resembling V(D)J recombination signal sequences. Between the TIRs reside tail-to-tail-oriented, intron-containing RAG1-like and RAG2-like genes. We demonstrate that ProtoRAG was recently active in the lancelet germline and that the lancelet RAG1/2-like proteins can mediate TIR-dependent transposon excision, host DNA recombination, transposition, and low-efficiency TIR rejoining using reaction mechanisms similar to those used by vertebrate RAGs. We propose that ProtoRAG represents a molecular "living fossil" of the long-sought RAG transposon.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Evidence of G.O.D.'s Miracle: Unearthing a RAG Transposon.Cell. 2016 Jun 30;166(1):11-2. doi: 10.1016/j.cell.2016.06.021. Cell. 2016. PMID: 27368095 Free PMC article.
References
-
- Agrawal A, Eastman QM, Schatz DG. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature. 1998;394:744–751. - PubMed
-
- Corneo B, Benmerah A, Villartay JP. A short peptide at the C terminus is responsible for the nuclear localization of RAG2. Eur J Immunol. 2002;32:2068–2073. - PubMed
-
- Corneo B, Wendland RL, Deriano L, Cui X, Klein IA, Wong SY, Arnal S, Holub AJ, Weller GR, Pancake BA, et al. Rag mutations reveal robust alternative end joining. Nature. 2007;449:483–486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

