Para-Phenylenediamine Induces Apoptotic Death of Melanoma Cells and Reduces Melanoma Tumour Growth in Mice
- PMID: 27293892
- PMCID: PMC4886052
- DOI: 10.1155/2016/3137010
Para-Phenylenediamine Induces Apoptotic Death of Melanoma Cells and Reduces Melanoma Tumour Growth in Mice
Abstract
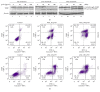
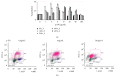
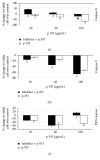
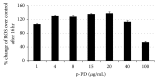
Melanoma is one of the most aggressive forms of cancer, usually resistant to standard chemotherapeutics. Despite a huge number of clinical trials, any success to find a chemotherapeutic agent that can effectively destroy melanoma is yet to be achieved. Para-phenylenediamine (p-PD) in the hair dyes is reported to purely serve as an external dyeing agent. Very little is known about whether p-PD has any effect on the melanin producing cells. We have demonstrated p-PD mediated apoptotic death of both human and mouse melanoma cells in vitro. Mouse melanoma tumour growth was also arrested by the apoptotic activity of intraperitoneal administration of p-PD with almost no side effects. This apoptosis is shown to occur primarily via loss of mitochondrial membrane potential (MMP), generation of reactive oxygen species (ROS), and caspase 8 activation. p-PD mediated apoptosis was also confirmed by the increase in sub-G0/G1 cell number. Thus, our experimental observation suggests that p-PD can be a potential less expensive candidate to be developed as a chemotherapeutic agent for melanoma.
Figures




Similar articles
-
4-chloro-1,2-phenylenediamine induces apoptosis in Mardin-Darby canine kidney cells via activation of caspases.Environ Toxicol. 2014 Jun;29(6):655-64. doi: 10.1002/tox.21792. Epub 2012 Jul 10. Environ Toxicol. 2014. PMID: 22778066
-
Apoptosis induced by para-phenylenediamine involves formation of ROS and activation of p38 and JNK in chang liver cells.Environ Toxicol. 2014 Sep;29(9):981-90. doi: 10.1002/tox.21828. Epub 2012 Nov 22. Environ Toxicol. 2014. PMID: 23172806
-
Induction of apoptosis by isoegomaketone from Perilla frutescens L. in B16 melanoma cells is mediated through ROS generation and mitochondrial-dependent, -independent pathway.Food Chem Toxicol. 2014 Mar;65:97-104. doi: 10.1016/j.fct.2013.12.031. Epub 2013 Dec 29. Food Chem Toxicol. 2014. PMID: 24380754
-
para-Phenylenediamine induces apoptosis through activation of reactive oxygen species-mediated mitochondrial pathway, and inhibition of the NF-κB, mTOR, and Wnt pathways in human urothelial cells.Environ Toxicol. 2017 Jan;32(1):265-277. doi: 10.1002/tox.22233. Epub 2016 Jan 19. Environ Toxicol. 2017. PMID: 26784575
-
Hydrogen peroxide induces cell death in human TRAIL-resistant melanoma through intracellular superoxide generation.Int J Oncol. 2013 Mar;42(3):863-72. doi: 10.3892/ijo.2013.1769. Epub 2013 Jan 10. Int J Oncol. 2013. PMID: 23314732
Cited by
-
Rhus semialata M. extract ameliorate para-phenylenediamine-induced toxicity in keratinocytes.Toxicol Rep. 2020 Dec 26;8:96-105. doi: 10.1016/j.toxrep.2020.12.020. eCollection 2021. Toxicol Rep. 2020. PMID: 33437652 Free PMC article.
-
Melanocytotoxic chemicals and their toxic mechanisms.Toxicol Res. 2022 Aug 22;38(4):417-435. doi: 10.1007/s43188-022-00144-2. eCollection 2022 Oct. Toxicol Res. 2022. PMID: 36277364 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

