An Optimized Injectable Hydrogel Scaffold Supports Human Dental Pulp Stem Cell Viability and Spreading
- PMID: 27294191
- PMCID: PMC4884792
- DOI: 10.1155/2016/7363579
An Optimized Injectable Hydrogel Scaffold Supports Human Dental Pulp Stem Cell Viability and Spreading
Abstract
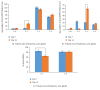
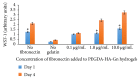
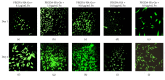
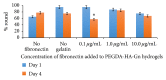
Introduction. HyStem-C™ is a commercially available injectable hydrogel composed of polyethylene glycol diacrylate (PEGDA), hyaluronan (HA), and gelatin (Gn). These components can be mechanically tuned to enhance cell viability and spreading. Methods. The concentration of PEGDA with an added disulfide bond (PEGSSDA) was varied from 0.5 to 8.0% (w/v) to determine the optimal concentration for injectable clinical application. We evaluated the cell viability of human dental pulp stem cells (hDPSCs) embedded in 2% (w/v) PEGSSDA-HA-Gn hydrogels. Volume ratios of HA : Gn from 100 : 0 to 25 : 75 were varied to encourage hDPSC spreading. Fibronectin (Fn) was added to our model to determine the effect of extracellular matrix protein concentration on hDPSC behavior. Results. Our preliminary data suggests that the hydrogel gelation time decreased as the PEGSSDA cross-linker concentration increased. The PEGSSDA-HA-Gn was biocompatible with hDPSCs, and increased ratios of HA : Gn enhanced cell viability for 14 days. Additionally, cell proliferation with added fibronectin increased significantly over time at concentrations of 1.0 and 10.0 μg/mL in PEGDA-HA-Gn hydrogels, while cell spreading significantly increased at Fn concentrations of 0.1 μg/mL. Conclusions. This study demonstrates that PEG-based injectable hydrogels maintain hDPSC viability and facilitate cell spreading, mainly in the presence of extracellular matrix (ECM) proteins.
Figures



Similar articles
-
Attachment and spreading of fibroblasts on an RGD peptide-modified injectable hyaluronan hydrogel.J Biomed Mater Res A. 2004 Feb 1;68(2):365-75. doi: 10.1002/jbm.a.20002. J Biomed Mater Res A. 2004. PMID: 14704979
-
Poly(ethylene glycol) diacrylate/hyaluronic acid semi-interpenetrating network compositions for 3-D cell spreading and migration.Acta Biomater. 2015 Mar;14:43-52. doi: 10.1016/j.actbio.2014.12.007. Epub 2014 Dec 15. Acta Biomater. 2015. PMID: 25523876 Free PMC article.
-
Disulfide-crosslinked hyaluronan-gelatin hydrogel films: a covalent mimic of the extracellular matrix for in vitro cell growth.Biomaterials. 2003 Sep;24(21):3825-34. doi: 10.1016/s0142-9612(03)00267-9. Biomaterials. 2003. PMID: 12818555
-
Fibronectin-loaded Collagen/Gelatin Hydrogel Is a Potent Signaling Biomaterial for Dental Pulp Regeneration.J Endod. 2021 Jul;47(7):1110-1117. doi: 10.1016/j.joen.2021.04.009. Epub 2021 Apr 19. J Endod. 2021. PMID: 33887309
-
A hybrid injectable hydrogel from hyperbranched PEG macromer as a stem cell delivery and retention platform for diabetic wound healing.Acta Biomater. 2018 Jul 15;75:63-74. doi: 10.1016/j.actbio.2018.05.039. Epub 2018 May 25. Acta Biomater. 2018. PMID: 29803782
Cited by
-
Innovative Approach in the Cryogenic Freezing Medium for Mesenchymal Stem Cells.Biomolecules. 2022 Apr 20;12(5):610. doi: 10.3390/biom12050610. Biomolecules. 2022. PMID: 35625538 Free PMC article.
-
Advances on Hydrogels for Oral Science Research.Gels. 2022 May 15;8(5):302. doi: 10.3390/gels8050302. Gels. 2022. PMID: 35621600 Free PMC article. Review.
-
The effect of polyethylenglycol gel on the delivery and osteogenic differentiation of homologous tooth germ-derived stem cells in a porcine model.Clin Oral Investig. 2021 May;25(5):3043-3057. doi: 10.1007/s00784-020-03625-6. Epub 2020 Oct 26. Clin Oral Investig. 2021. PMID: 33104929
-
Injectable Biomaterials for Dental Tissue Regeneration.Int J Mol Sci. 2020 May 13;21(10):3442. doi: 10.3390/ijms21103442. Int J Mol Sci. 2020. PMID: 32414077 Free PMC article. Review.
-
A narrative overview of utilizing biomaterials to recapitulate the salient regenerative features of dental-derived mesenchymal stem cells.Int J Oral Sci. 2021 Jun 30;13(1):22. doi: 10.1038/s41368-021-00126-4. Int J Oral Sci. 2021. PMID: 34193832 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

