Genetic variation and exercise-induced muscle damage: implications for athletic performance, injury and ageing
- PMID: 27294501
- PMCID: PMC4983298
- DOI: 10.1007/s00421-016-3411-1
Genetic variation and exercise-induced muscle damage: implications for athletic performance, injury and ageing
Abstract
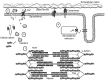
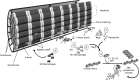
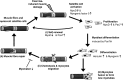
Prolonged unaccustomed exercise involving muscle lengthening (eccentric) actions can result in ultrastructural muscle disruption, impaired excitation-contraction coupling, inflammation and muscle protein degradation. This process is associated with delayed onset muscle soreness and is referred to as exercise-induced muscle damage. Although a certain amount of muscle damage may be necessary for adaptation to occur, excessive damage or inadequate recovery from exercise-induced muscle damage can increase injury risk, particularly in older individuals, who experience more damage and require longer to recover from muscle damaging exercise than younger adults. Furthermore, it is apparent that inter-individual variation exists in the response to exercise-induced muscle damage, and there is evidence that genetic variability may play a key role. Although this area of research is in its infancy, certain gene variations, or polymorphisms have been associated with exercise-induced muscle damage (i.e. individuals with certain genotypes experience greater muscle damage, and require longer recovery, following strenuous exercise). These polymorphisms include ACTN3 (R577X, rs1815739), TNF (-308 G>A, rs1800629), IL6 (-174 G>C, rs1800795), and IGF2 (ApaI, 17200 G>A, rs680). Knowing how someone is likely to respond to a particular type of exercise could help coaches/practitioners individualise the exercise training of their athletes/patients, thus maximising recovery and adaptation, while reducing overload-associated injury risk. The purpose of this review is to provide a critical analysis of the literature concerning gene polymorphisms associated with exercise-induced muscle damage, both in young and older individuals, and to highlight the potential mechanisms underpinning these associations, thus providing a better understanding of exercise-induced muscle damage.
Keywords: Creatine kinase; Delayed onset muscle soreness; Elderly; Exercise-induced muscle damage; Single nucleotide polymorphism.
Figures

References
-
- Ahmetov II, Naumov VA, Donnikov AE, Maciejewska-Karłowska A, Kostryukova ES, Larin AK, Maykova EV, Alexeev DG, Fedotovskaya ON, Generozov EV. SOD2 gene polymorphism and muscle damage markers in elite athletes. Free Radical Res. 2014;48(8):948–955. - PubMed
-
- Akimoto AK, Miranda-Vilela AL, Alves PC, Pereira LC, Lordelo GS, Hiragi Cde O, da Silva IC, Grisolia CK, Klautau-Guimaraes Mde N. Evaluation of gene polymorphisms in exercise-induced oxidative stress and damage. Free Radical Res. 2010;44(3):322–331. - PubMed
-
- Allen NE, Davey GK, Key TJ, Zhang S, Narod SA. Serum insulin-like growth factor I (IGF-I) concentration in men is not associated with the cytosine-adenosine repeat polymorphism of the IGF-I gene. Cancer Epidem Biomark. 2002;11(3):319–320. - PubMed
-
- Al-Shanti N, Stewart CE. Ca2+/calmodulin-dependent transcriptional pathways: potential mediators of skeletal muscle growth and development. Biol Rev. 2009;84(4):637–652. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

