Zeb2 is essential for Schwann cell differentiation, myelination and nerve repair
- PMID: 27294512
- PMCID: PMC4964942
- DOI: 10.1038/nn.4321
Zeb2 is essential for Schwann cell differentiation, myelination and nerve repair
Abstract
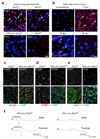
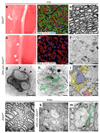
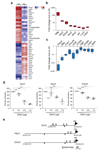
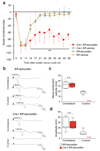
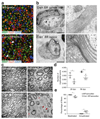
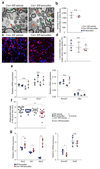
Schwann cell development and peripheral nerve myelination require the serial expression of transcriptional activators, such as Sox10, Oct6 (also called Scip or Pou3f1) and Krox20 (also called Egr2). Here we show that transcriptional repression, mediated by the zinc-finger protein Zeb2 (also known as Sip1), is essential for differentiation and myelination. Mice lacking Zeb2 in Schwann cells develop a severe peripheral neuropathy, caused by failure of axonal sorting and virtual absence of myelin membranes. Zeb2-deficient Schwann cells continuously express repressors of lineage progression. Moreover, genes for negative regulators of maturation such as Sox2 and Ednrb emerge as Zeb2 target genes, supporting its function as an 'inhibitor of inhibitors' in myelination control. When Zeb2 is deleted in adult mice, Schwann cells readily dedifferentiate following peripheral nerve injury and become repair cells. However, nerve regeneration and remyelination are both perturbed, demonstrating that Zeb2, although undetectable in adult Schwann cells, has a latent function throughout life.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
Zeb2: Inhibiting the inhibitors in Schwann cells.Neurogenesis (Austin). 2017 Feb 2;4(1):e1271495. doi: 10.1080/23262133.2016.1271495. eCollection 2017. Neurogenesis (Austin). 2017. PMID: 28203609 Free PMC article.
-
Transcription Factors and Coregulators in Schwann Cell Differentiation, Myelination, and Remyelination: Implications for Peripheral Neuropathy.J Neurosci Res. 2025 Jun;103(6):e70053. doi: 10.1002/jnr.70053. J Neurosci Res. 2025. PMID: 40452391 Review.
-
Dynamic regulation of Schwann cell enhancers after peripheral nerve injury.J Biol Chem. 2015 Mar 13;290(11):6937-50. doi: 10.1074/jbc.M114.622878. Epub 2015 Jan 22. J Biol Chem. 2015. PMID: 25614629 Free PMC article.
-
Signals regulating myelination in peripheral nerves and the Schwann cell response to injury.Curr Opin Neurobiol. 2013 Dec;23(6):1041-8. doi: 10.1016/j.conb.2013.06.010. Epub 2013 Jul 26. Curr Opin Neurobiol. 2013. PMID: 23896313 Free PMC article. Review.
-
Sox2 expression in Schwann cells inhibits myelination in vivo and induces influx of macrophages to the nerve.Development. 2017 Sep 1;144(17):3114-3125. doi: 10.1242/dev.150656. Epub 2017 Jul 25. Development. 2017. PMID: 28743796 Free PMC article.
Cited by
-
Targeted chromatin conformation analysis identifies novel distal neural enhancers of ZEB2 in pluripotent stem cell differentiation.Hum Mol Genet. 2020 Aug 29;29(15):2535-2550. doi: 10.1093/hmg/ddaa141. Hum Mol Genet. 2020. PMID: 32628253 Free PMC article.
-
CTCF-mediated chromatin looping in EGR2 regulation and SUZ12 recruitment critical for peripheral myelination and repair.Nat Commun. 2020 Aug 17;11(1):4133. doi: 10.1038/s41467-020-17955-2. Nat Commun. 2020. PMID: 32807777 Free PMC article.
-
Schwann cell development, maturation and regeneration: a focus on classic and emerging intracellular signaling pathways.Neural Regen Res. 2017 Jul;12(7):1013-1023. doi: 10.4103/1673-5374.211172. Neural Regen Res. 2017. PMID: 28852375 Free PMC article. Review.
-
Zeb2: Inhibiting the inhibitors in Schwann cells.Neurogenesis (Austin). 2017 Feb 2;4(1):e1271495. doi: 10.1080/23262133.2016.1271495. eCollection 2017. Neurogenesis (Austin). 2017. PMID: 28203609 Free PMC article.
-
Implications of Schwann Cells Biomechanics and Mechanosensitivity for Peripheral Nervous System Physiology and Pathophysiology.Front Mol Neurosci. 2017 Oct 25;10:345. doi: 10.3389/fnmol.2017.00345. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29118694 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

